当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Late-Stage Lead Diversification Coupled with Quantitative Nuclear Magnetic Resonance Spectroscopy to Identify New Structure-Activity Relationship Vectors at Nanomole-Scale Synthesis: Application to Loratadine, a Human Histamine H1 Receptor Inverse Agonist.
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-05-28 , DOI: 10.1021/acs.jmedchem.0c00483 Manjinder S Lall 1 , Asser Bassyouni 2 , James Bradow 1 , Maria Brown 1 , Mark Bundesmann 1 , Jinshan Chen 1 , Gregory Ciszewski 1 , Anne E Hagen 1 , Dennis Hyek 3 , Stephen Jenkinson 2 , Bo Liu 3 , R Scott Obach 1 , Senliang Pan 1 , Usa Reilly 1 , Neal Sach 2 , Daniel J Smaltz 4 , Douglas K Spracklin 1 , Jeremy Starr 1 , Melissa Wagenaar 1 , Gregory S Walker 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-05-28 , DOI: 10.1021/acs.jmedchem.0c00483 Manjinder S Lall 1 , Asser Bassyouni 2 , James Bradow 1 , Maria Brown 1 , Mark Bundesmann 1 , Jinshan Chen 1 , Gregory Ciszewski 1 , Anne E Hagen 1 , Dennis Hyek 3 , Stephen Jenkinson 2 , Bo Liu 3 , R Scott Obach 1 , Senliang Pan 1 , Usa Reilly 1 , Neal Sach 2 , Daniel J Smaltz 4 , Douglas K Spracklin 1 , Jeremy Starr 1 , Melissa Wagenaar 1 , Gregory S Walker 1
Affiliation
|
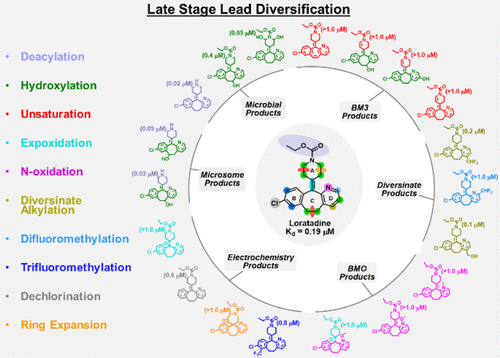
An experimental approach is described for late-stage lead diversification of frontrunner drug candidates using nanomole-scale amounts of lead compounds for structure–activity relationship development. The process utilizes C–H bond activation methods to explore chemical space by transforming candidates into newly functionalized leads. A key to success is the utilization of microcryoprobe nuclear magnetic resonance (NMR) spectroscopy, which permits the use of low amounts of lead compounds (1–5 μmol). The approach delivers multiple analogues from a single lead at nanomole-scale amounts as DMSO-d6 stock solutions with a known structure and concentration for in vitro pharmacology and absorption, distribution, metabolism, and excretion testing. To demonstrate the feasibility of this approach, we have used the antihistamine agent loratadine (1). Twenty-six analogues of loratadine were isolated and fully characterized by NMR. Informative SAR analogues were identified, which display potent affinity for the human histamine H1 receptor and improved metabolic stability.
中文翻译:

后期铅多样化与定量核磁共振波谱结合以在纳米级规模合成中识别新的结构-活性关系向量:应用于人组胺H1受体逆激动剂氯雷他定。
描述了一种实验方法,该方法使用纳米分子量的铅化合物用于领先分子候选药物的后期铅多样化,以发展结构-活性关系。该过程利用C–H键激活方法,通过将候选物转化为新功能化的铅来探索化学空间。成功的关键是利用微探针核磁共振(NMR)光谱技术,该技术可使用少量的铅化合物(1-5μmol)。该方法以纳摩尔级的量从单个铅中提供多种类似物,如DMSO- d 6储液,具有体外已知的结构和浓度药理学和吸收,分布,代谢和排泄测试。为了证明这种方法的可行性,我们使用了抗组胺药氯雷他定(1)。分离出26种氯雷他定类似物,并通过NMR进行了全面表征。鉴定出信息性的SAR类似物,其对人组胺H 1受体显示出强大的亲和力,并改善了代谢稳定性。
更新日期:2020-07-09
中文翻译:

后期铅多样化与定量核磁共振波谱结合以在纳米级规模合成中识别新的结构-活性关系向量:应用于人组胺H1受体逆激动剂氯雷他定。
描述了一种实验方法,该方法使用纳米分子量的铅化合物用于领先分子候选药物的后期铅多样化,以发展结构-活性关系。该过程利用C–H键激活方法,通过将候选物转化为新功能化的铅来探索化学空间。成功的关键是利用微探针核磁共振(NMR)光谱技术,该技术可使用少量的铅化合物(1-5μmol)。该方法以纳摩尔级的量从单个铅中提供多种类似物,如DMSO- d 6储液,具有体外已知的结构和浓度药理学和吸收,分布,代谢和排泄测试。为了证明这种方法的可行性,我们使用了抗组胺药氯雷他定(1)。分离出26种氯雷他定类似物,并通过NMR进行了全面表征。鉴定出信息性的SAR类似物,其对人组胺H 1受体显示出强大的亲和力,并改善了代谢稳定性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号