当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bioisosteric Replacement of Arylamide-Linked Spine Residues with N-Acylhydrazones and Selenophenes as a Design Strategy to Novel Dibenzosuberone Derivatives as Type I 1/2 p38α MAP Kinase Inhibitors.
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-05-28 , DOI: 10.1021/acs.jmedchem.0c00508 Júlia G B Pedreira 1, 2 , Philipp Nahidino 3 , Mark Kudolo 3 , Tatu Pantsar 3, 4 , Benedict-Tilman Berger 5, 6 , Michael Forster 3 , Stefan Knapp 5, 6 , Stefan Laufer 3, 7, 8 , Eliezer J Barreiro 1, 2
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-05-28 , DOI: 10.1021/acs.jmedchem.0c00508 Júlia G B Pedreira 1, 2 , Philipp Nahidino 3 , Mark Kudolo 3 , Tatu Pantsar 3, 4 , Benedict-Tilman Berger 5, 6 , Michael Forster 3 , Stefan Knapp 5, 6 , Stefan Laufer 3, 7, 8 , Eliezer J Barreiro 1, 2
Affiliation
|
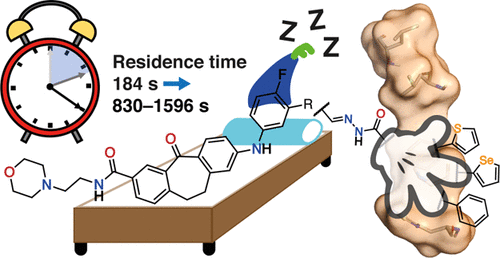
The recent disclosure of type I 1/2 inhibitors for p38α MAPK demonstrated how the stabilization of the R-spine can be used as a strategy to greatly increase the target residence time (TRT) of inhibitors. Herein, for the first time, we describe N-acylhydrazone and selenophene residues as spine motifs, yielding metabolically stable inhibitors with high potency on enzymatic, NanoBRET, and whole blood assays, improved metabolic stability, and prolonged TRT.
中文翻译:

N-酰基hydr和硒烯的芳族酰胺连接的脊柱残基的生物立体置换作为一种设计策略,以新颖的二苯并亚砜衍生物作为I 1/2p38αMAP激酶抑制剂。
p38αMAPK的I 1/2型抑制剂的最新披露表明,如何将R脊柱的稳定化用作增加抑制剂的目标停留时间(TRT)的策略。在本文中,我们首次将N-酰基and和硒基残基描述为脊柱基序,产生了对酶,NanoBRET和全血检测具有高效效的代谢稳定抑制剂,改善了代谢稳定性,并延长了TRT。
更新日期:2020-07-09
中文翻译:

N-酰基hydr和硒烯的芳族酰胺连接的脊柱残基的生物立体置换作为一种设计策略,以新颖的二苯并亚砜衍生物作为I 1/2p38αMAP激酶抑制剂。
p38αMAPK的I 1/2型抑制剂的最新披露表明,如何将R脊柱的稳定化用作增加抑制剂的目标停留时间(TRT)的策略。在本文中,我们首次将N-酰基and和硒基残基描述为脊柱基序,产生了对酶,NanoBRET和全血检测具有高效效的代谢稳定抑制剂,改善了代谢稳定性,并延长了TRT。











































 京公网安备 11010802027423号
京公网安备 11010802027423号