当前位置:
X-MOL 学术
›
Environ. Prog. Sustain. Energy
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High adsorption capacity of nitrobenzene from aqueous solution using activated carbons prepared from vegetable waste
Environmental Progress & Sustainable Energy ( IF 2.1 ) Pub Date : 2020-05-28 , DOI: 10.1002/ep.13463 Zoubida Kecira 1 , Oumessaâd Benturki 1 , Asma Benturki 1 , Mounir Daoud 1, 2 , Pierre Girods 3
Environmental Progress & Sustainable Energy ( IF 2.1 ) Pub Date : 2020-05-28 , DOI: 10.1002/ep.13463 Zoubida Kecira 1 , Oumessaâd Benturki 1 , Asma Benturki 1 , Mounir Daoud 1, 2 , Pierre Girods 3
Affiliation
|
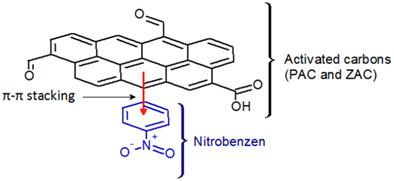
The present study reports the preparation of chemically activated carbons (PAC and ZAC) from apricot stones using phosphoric acid and zinc chloride, respectively. The potential use of the prepared carbons, as adsorbents, and the effect of chemical activation on the surface properties and adsorptive mechanism of nitrobenzene (NB) were investigated. The physicochemical properties of the obtained materials were characterized by Nitrogen adsorption–desorption isotherms, Scanning Electron Microscope, FTIR Spectroscopy, EDX analysis, Boehm titration, and pH of zero charge. The results show that the prepared samples present high apparent surface areas (1,382 and 1,111 m2/g for PAC and ZAC, respectively) and pores volumes with the presence of various functional groups. It was found that pseudo‐second model was the most suitable for the fitting of the experimental kinetic data and the intraparticle diffusion was not the unique rate‐controlling stage. The adsorption isotherms were well described by the Langmuir and the Freundlich models. According to the Langmuir model, the maximum adsorption capacity of PAC and ZAC were about 476.2 and 490.2 mg/g, respectively. The thermodynamic parameters showed the spontaneity, the exothermic nature, and the decrease in the randomness of the adsorption of nitrobenzene. Chemical regeneration showed that the prepared samples could be used for four time desorption–adsorption cycles with good efficiency for NB removal, indicating that the prepared carbons could be used as a low‐cost alternative to commercial activated carbon for the removal of nitrobenzene from wastewater.
中文翻译:

使用植物废料制得的活性炭对水溶液中硝基苯的高吸附能力
本研究报告了分别使用磷酸和氯化锌从杏石中制备化学活性炭(PAC和ZAC)。研究了制备的碳作为吸附剂的潜在用途,以及化学活化对硝基苯(NB)的表面性质和吸附机理的影响。通过氮气吸附-解吸等温线,扫描电子显微镜,FTIR光谱,EDX分析,Boehm滴定和零电荷的pH表征了所获得材料的理化性质。结果表明,制备的样品具有较高的表观表面积(1,382和1,111 m 2/ g分别对应于PAC和ZAC)和孔体积(存在各种官能团)。发现伪秒模型最适合实验动力学数据的拟合,粒子内扩散不是唯一的速率控制阶段。Langmuir和Freundlich模型很好地描述了吸附等温线。根据Langmuir模型,PAC和ZAC的最大吸附容量分别约为476.2和490.2 mg / g。热力学参数显示出自发性,放热性质和硝基苯吸附随机性的降低。化学再生表明,所制备的样品可用于四个时间的解吸-吸附循环,具有良好的NB去除效率,
更新日期:2020-05-28
中文翻译:

使用植物废料制得的活性炭对水溶液中硝基苯的高吸附能力
本研究报告了分别使用磷酸和氯化锌从杏石中制备化学活性炭(PAC和ZAC)。研究了制备的碳作为吸附剂的潜在用途,以及化学活化对硝基苯(NB)的表面性质和吸附机理的影响。通过氮气吸附-解吸等温线,扫描电子显微镜,FTIR光谱,EDX分析,Boehm滴定和零电荷的pH表征了所获得材料的理化性质。结果表明,制备的样品具有较高的表观表面积(1,382和1,111 m 2/ g分别对应于PAC和ZAC)和孔体积(存在各种官能团)。发现伪秒模型最适合实验动力学数据的拟合,粒子内扩散不是唯一的速率控制阶段。Langmuir和Freundlich模型很好地描述了吸附等温线。根据Langmuir模型,PAC和ZAC的最大吸附容量分别约为476.2和490.2 mg / g。热力学参数显示出自发性,放热性质和硝基苯吸附随机性的降低。化学再生表明,所制备的样品可用于四个时间的解吸-吸附循环,具有良好的NB去除效率,











































 京公网安备 11010802027423号
京公网安备 11010802027423号