当前位置:
X-MOL 学术
›
Eur. J. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Juxtacrine signalling via Notch and ErbB receptors in the switch to fate commitment of bone marrow-derived Schwann cells.
European Journal of Neuroscience ( IF 2.7 ) Pub Date : 2020-05-27 , DOI: 10.1111/ejn.14837 Graham Ka-Hon Shea 1 , Evelyn Wing-Yin Tai 1, 2 , Katherine Ho-Yan Leung 2 , Alan Kwan-Long Mung 1, 2 , Maximilian Tak-Sui Li 2, 3 , Alex Yat-Ping Tsui 2, 3 , Anthony Kin-Wai Tam 2, 3 , Daisy Kwok-Yan Shum 2, 3, 4 , Ying-Shing Chan 2, 3, 4
European Journal of Neuroscience ( IF 2.7 ) Pub Date : 2020-05-27 , DOI: 10.1111/ejn.14837 Graham Ka-Hon Shea 1 , Evelyn Wing-Yin Tai 1, 2 , Katherine Ho-Yan Leung 2 , Alan Kwan-Long Mung 1, 2 , Maximilian Tak-Sui Li 2, 3 , Alex Yat-Ping Tsui 2, 3 , Anthony Kin-Wai Tam 2, 3 , Daisy Kwok-Yan Shum 2, 3, 4 , Ying-Shing Chan 2, 3, 4
Affiliation
|
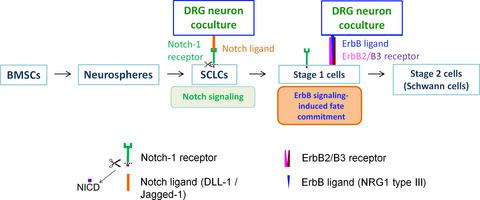
The phenotypic instability of adult tissue‐derived Schwann cell‐like cells (SCLCs) as revealed upon withdrawal of glia‐inducing culture supplements limits their clinical utility for cell therapy and disease modelling. We previously overcame this limitation by co‐culturing bone marrow‐derived SCLCs with neurons purified from developing rat and subsequently human sensory neurons such that direct contact between cell types accomplished the cell‐intrinsic switch to the Schwann cell fate. Here, our search for juxtacrine instructive signals found both Notch ligands and neuregulin‐1 type III localized on the surface of DRG neurons via live cell immunocytochemistry. Bypassing ligand‐induced release of the Notch intracellular domain (NICD) by transient transfection of SCLCs with the pAdlox/V5‐His‐NICD construct was shown to upregulate ErbB2/3. Interaction of ErbB2/3 with neuregulin‐1 type III (NRG1 type III) as presented on neurons then mediated the switch to the Schwann cell fate as demonstrated by expression of S100β/p75/ Sox10/Krox20. In contrast, treatment of cocultures with γ‐secretase inhibitor perturbed Notch signalling in SCLCs and consequently deterred both upregulation of ErbB2/3 and the transition to the Schwann cell fate. Taken together, juxtacrine signalling via Notch is key to the upregulation of ErbB receptors for neuregulin‐driven commitment of SCLCs to the Schwann cell fate.
中文翻译:

通过Notch和ErbB受体的邻分泌分泌信号转换成源自骨髓的雪旺细胞的命运。
成年组织来源的雪旺氏细胞样细胞(SCLC)的表型不稳定性,在撤回胶质细胞诱导培养补充剂后发现,限制了它们在细胞治疗和疾病建模中的临床效用。我们以前通过将骨髓来源的SCLC与从发育中的大鼠中提纯的神经元和随后的人类感觉神经元共培养来克服了这一局限,从而使细胞类型之间的直接接触实现了细胞内在转换为施旺细胞的命运。在这里,我们对近分泌指示性信号的搜索发现,Notch配体和neuregulin-1 III型都通过活细胞免疫细胞化学定位在DRG神经元表面。用pAdlox / V5-His-NICD构建体瞬时转染SCLC绕过配体诱导的Notch细胞内结构域(NICD)释放可上调ErbB2 / 3。如在S100β/ p75 / Sox10 / Krox20的表达所示,神经元上出现的ErbB2 / 3与神经调节素-1型III(NRG1型III)的相互作用随后介导了向雪旺氏细胞命运的转换。相比之下,用γ-分泌酶抑制剂对共培养物的处理会干扰SCLC中的Notch信号,从而阻止ErbB2 / 3的上调和向Schwann细胞命运的过渡。综上所述,通过Notch发出的邻分泌分泌信号是上调神经胶蛋白驱动的SCLC对Schwann细胞命运的承诺的ErbB受体上调的关键。γ-分泌酶抑制剂对共培养物的治疗会扰乱SCLC中的Notch信号,因此阻止了ErbB2 / 3的上调和向Schwann细胞命运的过渡。综上所述,通过Notch发出的邻分泌分泌信号是上调神经胶蛋白驱动的SCLC对Schwann细胞命运的承诺的ErbB受体上调的关键。γ-分泌酶抑制剂对共培养物的治疗会扰乱SCLC中的Notch信号,因此阻止了ErbB2 / 3的上调和向Schwann细胞命运的过渡。综上所述,通过Notch发出的邻分泌分泌信号是上调神经胶蛋白驱动的SCLC对Schwann细胞命运的承诺的ErbB受体上调的关键。
更新日期:2020-05-27
中文翻译:

通过Notch和ErbB受体的邻分泌分泌信号转换成源自骨髓的雪旺细胞的命运。
成年组织来源的雪旺氏细胞样细胞(SCLC)的表型不稳定性,在撤回胶质细胞诱导培养补充剂后发现,限制了它们在细胞治疗和疾病建模中的临床效用。我们以前通过将骨髓来源的SCLC与从发育中的大鼠中提纯的神经元和随后的人类感觉神经元共培养来克服了这一局限,从而使细胞类型之间的直接接触实现了细胞内在转换为施旺细胞的命运。在这里,我们对近分泌指示性信号的搜索发现,Notch配体和neuregulin-1 III型都通过活细胞免疫细胞化学定位在DRG神经元表面。用pAdlox / V5-His-NICD构建体瞬时转染SCLC绕过配体诱导的Notch细胞内结构域(NICD)释放可上调ErbB2 / 3。如在S100β/ p75 / Sox10 / Krox20的表达所示,神经元上出现的ErbB2 / 3与神经调节素-1型III(NRG1型III)的相互作用随后介导了向雪旺氏细胞命运的转换。相比之下,用γ-分泌酶抑制剂对共培养物的处理会干扰SCLC中的Notch信号,从而阻止ErbB2 / 3的上调和向Schwann细胞命运的过渡。综上所述,通过Notch发出的邻分泌分泌信号是上调神经胶蛋白驱动的SCLC对Schwann细胞命运的承诺的ErbB受体上调的关键。γ-分泌酶抑制剂对共培养物的治疗会扰乱SCLC中的Notch信号,因此阻止了ErbB2 / 3的上调和向Schwann细胞命运的过渡。综上所述,通过Notch发出的邻分泌分泌信号是上调神经胶蛋白驱动的SCLC对Schwann细胞命运的承诺的ErbB受体上调的关键。γ-分泌酶抑制剂对共培养物的治疗会扰乱SCLC中的Notch信号,因此阻止了ErbB2 / 3的上调和向Schwann细胞命运的过渡。综上所述,通过Notch发出的邻分泌分泌信号是上调神经胶蛋白驱动的SCLC对Schwann细胞命运的承诺的ErbB受体上调的关键。











































 京公网安备 11010802027423号
京公网安备 11010802027423号