当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diphenylene Iodonium Is a Noncovalent MAO Inhibitor: A Biochemical and Structural Analysis.
ChemMedChem ( IF 3.6 ) Pub Date : 2020-05-27 , DOI: 10.1002/cmdc.202000264 Luca G Iacovino 1 , Joana Reis 1 , Antonello Mai 2 , Claudia Binda 1 , Andrea Mattevi 1
ChemMedChem ( IF 3.6 ) Pub Date : 2020-05-27 , DOI: 10.1002/cmdc.202000264 Luca G Iacovino 1 , Joana Reis 1 , Antonello Mai 2 , Claudia Binda 1 , Andrea Mattevi 1
Affiliation
|
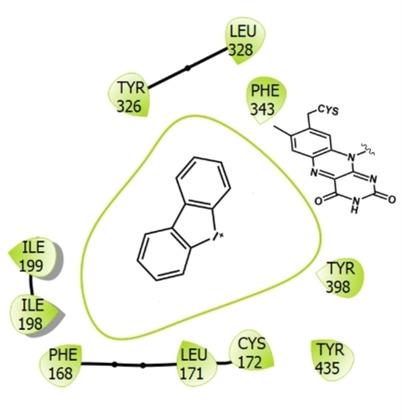
Diphenylene iodonium (DPI) is known for its inhibitory activities against many flavin‐ and heme‐dependent enzymes, and is often used as an NADPH oxidase inhibitor. We probed the efficacy of DPI on two well‐known drug targets, the human monoamine oxidases MAO A and B. UV‐visible spectrophotometry and steady‐state kinetics experiments demonstrate that DPI acts as a competitive and reversible MAO inhibitor with K i values of 1.7 and 0.3 μM for MAO A and MAO B, respectively. Elucidation of the crystal structure of human MAO B bound to the inhibitor revealed that DPI binds deeply in the active‐site cavity to establish multiple hydrophobic interactions with the surrounding side chains and the flavin. These data prove that DPI is a genuine MAO inhibitor and that the inhibition mechanism does not involve a reaction with the reduced flavin. This binding and inhibitory activity against the MAOs, two major reactive oxygen species (ROS)‐producing enzymes, will have to be carefully considered when interpreting experiments that rely on DPI for target validation and chemical biology studies on ROS functions.
中文翻译:

联苯碘鎓是一种非共价的MAO抑制剂:生化和结构分析。
联苯碘鎓(DPI)以其对许多黄素和血红素依赖性酶的抑制活性而闻名,通常用作NADPH氧化酶抑制剂。我们探讨了DPI在两个著名的药物靶标上的作用,即人单胺氧化酶MAO A和B。紫外可见分光光度法和稳态动力学实验表明DPI与K i一起起竞争性和可逆性MAO抑制剂的作用MAO A和MAO B的值分别为1.7和0.3μM。阐明了与抑制剂结合的人MAO B的晶体结构,发现DPI在活性位腔中深深地结合,与周围的侧链和黄素建立了多个疏水相互作用。这些数据证明DPI是真正的MAO抑制剂,并且抑制机理不涉及与还原的黄素的反应。在解释依赖于DPI进行目标验证和对ROS功能进行化学生物学研究的实验时,必须仔细考虑对MAOs的结合和抑制活性,MAOs是两种主要的产生活性氧的酶(ROS)。
更新日期:2020-08-05
中文翻译:

联苯碘鎓是一种非共价的MAO抑制剂:生化和结构分析。
联苯碘鎓(DPI)以其对许多黄素和血红素依赖性酶的抑制活性而闻名,通常用作NADPH氧化酶抑制剂。我们探讨了DPI在两个著名的药物靶标上的作用,即人单胺氧化酶MAO A和B。紫外可见分光光度法和稳态动力学实验表明DPI与K i一起起竞争性和可逆性MAO抑制剂的作用MAO A和MAO B的值分别为1.7和0.3μM。阐明了与抑制剂结合的人MAO B的晶体结构,发现DPI在活性位腔中深深地结合,与周围的侧链和黄素建立了多个疏水相互作用。这些数据证明DPI是真正的MAO抑制剂,并且抑制机理不涉及与还原的黄素的反应。在解释依赖于DPI进行目标验证和对ROS功能进行化学生物学研究的实验时,必须仔细考虑对MAOs的结合和抑制活性,MAOs是两种主要的产生活性氧的酶(ROS)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号