当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ruthenium(II)‐catalyzed Monohydroalkylation of α,β‐Unsaturated Ketones with N‐Acyl Pyrroles using a C−H Activation Strategy
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-05-28 , DOI: 10.1002/ajoc.202000264 Weiqiang Chen 1 , Hui‐Jing Li 1, 2 , Wen‐Yu Lu 1 , Yan‐Chao Wu 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-05-28 , DOI: 10.1002/ajoc.202000264 Weiqiang Chen 1 , Hui‐Jing Li 1, 2 , Wen‐Yu Lu 1 , Yan‐Chao Wu 1
Affiliation
|
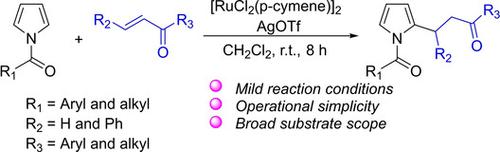
A ruthenium(II)‐catalyzed Michael addition of N‐acyl pyrroles to α,β‐unsaturated ketones has been developed by using a C−H activation strategy. The key to this selective reaction is to use an acyl group as an effective chelating group. The use of AgOTf remarkably promoted the protonolysis process and thereby facilitated the Michael addition reaction. Monoalkylated pyrroles could be selectively synthesized by controlling the ratio of α,β‐unsaturated ketones to N‐acyl pyrroles.
中文翻译:

钌(II)通过CH活化策略用N-酰基吡咯催化α,β-不饱和酮的单氢烷基化
通过使用CH活化策略,已经开发了钌(II)催化的N-酰基吡咯基团向N,β-不饱和酮的迈克尔加成反应。该选择性反应的关键是使用酰基作为有效的螯合基团。AgOTf的使用显着促进了质子分解过程,从而促进了迈克尔加成反应。可以通过控制α,β-不饱和酮与N-酰基吡咯的比例来选择性合成单烷基吡咯。
更新日期:2020-05-28
中文翻译:

钌(II)通过CH活化策略用N-酰基吡咯催化α,β-不饱和酮的单氢烷基化
通过使用CH活化策略,已经开发了钌(II)催化的N-酰基吡咯基团向N,β-不饱和酮的迈克尔加成反应。该选择性反应的关键是使用酰基作为有效的螯合基团。AgOTf的使用显着促进了质子分解过程,从而促进了迈克尔加成反应。可以通过控制α,β-不饱和酮与N-酰基吡咯的比例来选择性合成单烷基吡咯。











































 京公网安备 11010802027423号
京公网安备 11010802027423号