Structure ( IF 4.4 ) Pub Date : 2020-05-28 , DOI: 10.1016/j.str.2020.05.004 Andreyah L Pope 1 , Omar B Sanchez-Reyes 1 , Kieron South 2 , Ekaterina Zaitseva 3 , Martine Ziliox 1 , Reiner Vogel 3 , Philip J Reeves 2 , Steven O Smith 1
|
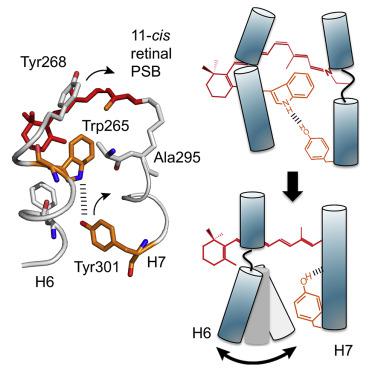
Despite high-resolution crystal structures of both inactive and active G protein-coupled receptors (GPCRs), it is still not known how ligands trigger the large structural change on the intracellular side of the receptor since the conformational changes that occur within the extracellular ligand-binding region upon activation are subtle. Here, we use solid-state NMR and Fourier transform infrared spectroscopy on rhodopsin to show that Trp2656.48 within the CWxP motif on transmembrane helix H6 constrains a proline hinge in the inactive state, suggesting that activation results in unraveling of the H6 backbone within this motif, a local change in dynamics that allows helix H6 to swing outward. Notably, Tyr3017.48 within activation switch 2 appears to mimic the negative allosteric sodium ion found in other family A GPCRs, a finding that is broadly relevant to the mechanism of receptor activation.
中文翻译:

保守的脯氨酸铰链介导视紫质的螺旋动力学和活化。
尽管非活性和活性 G 蛋白偶联受体 (GPCR) 都有高分辨率晶体结构,但仍然不清楚配体如何触发受体细胞内的巨大结构变化,因为细胞外配体中发生的构象变化 -激活时的结合区域是微妙的。在这里,我们在视紫质上使用固态 NMR 和傅里叶变换红外光谱来显示跨膜螺旋 H6 上 CWxP 基序内的Trp265 6.48限制处于非活性状态的脯氨酸铰链,这表明激活导致该基序内 H6 主链的解开,动态的局部变化,使螺旋 H6 向外摆动。值得注意的是,Tyr301 7.48 激活开关 2 中的 2 似乎模拟了在其他 A 家族 GPCR 中发现的负变构钠离子,这一发现与受体激活机制广泛相关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号