当前位置:
X-MOL 学术
›
J. Mol. Struct.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photophysical, theoretical, pharmacogenomics and biological studies of synthesized new symmetrical diol schiff base and 4-arylidene curcumin monomers
Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.molstruc.2020.128491 A. Subramani , L. Benazir Ali , V. Rosi , T.K. Shabeer
Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.molstruc.2020.128491 A. Subramani , L. Benazir Ali , V. Rosi , T.K. Shabeer
|
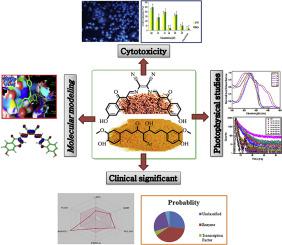
Abstract This research work was emphasized about two different new symmetrical diol monomers: (i) Schiff base monomers (1a-1d) and (ii) 4-Arylidine curcumin monomers (2a, 2b). Formulations of all monomers have been deduced by elemental analysis and spectral data (FT IR, 1H NMR, 13C NMR, and GC/MS spectroscopy). Investigations on photophysical properties of monomers have been studied in THF using steady state absorption, fluorescence, and excited-state time-resolved fluorescence techniques. The chemical stability of title compounds was accomplished by DFT theory using B3LYP/3-21G∗++ basis sets. All the monomers were strongly interacted with HSA a compound 2a higher negative docking score is considered as more potent. The monomers (1a-1d & 2a, 2b) which that approved through docking profiles have examined their potency against A549 and cervical HeLa cancer and normal monkey kidney cells by in vitro. The compound 2a showed a highest potency against the A549 cell line with the IC50 value 18.81%. These results matched well with the results observed from molecular docking studies. Additionally, the proposed set of monomers optimized by virtual ADMET screening and molecular simulation methods. Hence, the overall present study paves the way for designing new drugs for anticancer and antimycobacterial activities with elevated inhibitory potency.
中文翻译:

合成的新型对称二醇席夫碱和 4-亚芳基姜黄素单体的光物理、理论、药物基因组学和生物学研究
摘要 这项研究工作着重于两种不同的新型对称二醇单体:(i) 席夫碱单体 (1a-1d) 和 (ii) 4-芳烷姜黄素单体 (2a, 2b)。已通过元素分析和光谱数据(FT IR、1H NMR、13C NMR 和 GC/MS 光谱)推导出所有单体的配方。已经使用稳态吸收、荧光和激发态时间分辨荧光技术在 THF 中研究了单体的光物理特性。标题化合物的化学稳定性是通过 DFT 理论使用 B3LYP/3-21G*++ 基组完成的。所有单体都与 HSA 强烈相互作用,化合物 2a 较高的负对接分数被认为更有效。单体 (1a-1d & 2a, 2b) 通过对接配置文件批准的已在体外检查了它们对 A549 和宫颈 HeLa 癌以及正常猴肾细胞的效力。化合物2a对A549细胞系显示出最高效力,IC50值为18.81%。这些结果与从分子对接研究中观察到的结果非常吻合。此外,提议的一组单体通过虚拟 ADMET 筛选和分子模拟方法进行了优化。因此,目前的整体研究为设计具有高抑制效力的抗癌和抗分枝杆菌活性新药铺平了道路。通过虚拟 ADMET 筛选和分子模拟方法优化的拟议单体集。因此,目前的整体研究为设计具有高抑制效力的抗癌和抗分枝杆菌活性新药铺平了道路。通过虚拟 ADMET 筛选和分子模拟方法优化的拟议单体集。因此,目前的整体研究为设计具有高抑制效力的抗癌和抗分枝杆菌活性新药铺平了道路。
更新日期:2020-10-01
中文翻译:

合成的新型对称二醇席夫碱和 4-亚芳基姜黄素单体的光物理、理论、药物基因组学和生物学研究
摘要 这项研究工作着重于两种不同的新型对称二醇单体:(i) 席夫碱单体 (1a-1d) 和 (ii) 4-芳烷姜黄素单体 (2a, 2b)。已通过元素分析和光谱数据(FT IR、1H NMR、13C NMR 和 GC/MS 光谱)推导出所有单体的配方。已经使用稳态吸收、荧光和激发态时间分辨荧光技术在 THF 中研究了单体的光物理特性。标题化合物的化学稳定性是通过 DFT 理论使用 B3LYP/3-21G*++ 基组完成的。所有单体都与 HSA 强烈相互作用,化合物 2a 较高的负对接分数被认为更有效。单体 (1a-1d & 2a, 2b) 通过对接配置文件批准的已在体外检查了它们对 A549 和宫颈 HeLa 癌以及正常猴肾细胞的效力。化合物2a对A549细胞系显示出最高效力,IC50值为18.81%。这些结果与从分子对接研究中观察到的结果非常吻合。此外,提议的一组单体通过虚拟 ADMET 筛选和分子模拟方法进行了优化。因此,目前的整体研究为设计具有高抑制效力的抗癌和抗分枝杆菌活性新药铺平了道路。通过虚拟 ADMET 筛选和分子模拟方法优化的拟议单体集。因此,目前的整体研究为设计具有高抑制效力的抗癌和抗分枝杆菌活性新药铺平了道路。通过虚拟 ADMET 筛选和分子模拟方法优化的拟议单体集。因此,目前的整体研究为设计具有高抑制效力的抗癌和抗分枝杆菌活性新药铺平了道路。











































 京公网安备 11010802027423号
京公网安备 11010802027423号