Journal of Catalysis ( IF 6.5 ) Pub Date : 2020-05-28 , DOI: 10.1016/j.jcat.2020.05.022 Casey B. Jones , Ishant Khurana , Siddarth H. Krishna , Arthur J. Shih , W. Nicholas Delgass , Jeffrey T. Miller , Fabio H. Ribeiro , William F. Schneider , Rajamani Gounder
|
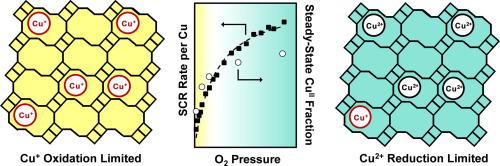
At low temperatures (<523 K), the selective catalytic reduction (SCR) of NO with NH3 on Cu-exchanged zeolites occurs via elementary steps catalyzed by NH3-solvated Cu ions, which are reactive intermediates in a CuII/CuI redox cycle. SCR rates are typically measured under “standard” reaction conditions, in which O2 is the oxidant and present at partial pressures (~10 kPa O2) that cause both single-site CuII reduction and dual-site CuI oxidation to behave as kinetically relevant steps. As a result, “standard” SCR rates (per g) transition from a second-order to a first-order dependence on isolated Cu content (per g) among Cu-CHA zeolites with increasing Cu content and thus spatial density (Si/Al = 15, Cu/Al = 0.08–0.37), as dual-site CuI oxidation steps limit SCR rates to lesser extents. On a given Cu-CHA catalyst, SCR rates (per Cu, 473 K) show a Langmuirian dependence on dioxygen pressure when varied widely (0–60 kPa O2), enabling the isolation of first-order or zero-order kinetic regimes with respect to O2. These kinetic regimes respectively correspond to limiting conditions in which either CuI oxidation or CuII reduction becomes the dominant kinetically relevant step, consistent with in operando X-ray absorption spectra. First-order rate constants (per Cu) increase approximately linearly with Cu density, reflecting the dual-site requirement of O2-assisted CuI-oxidation steps. Zero-order rate constants (per Cu) increase more gradually with Cu density, in part reflecting the increasing fraction of isolated Cu ions that are able to form binuclear intermediates and thus participate in SCR turnovers. Combining steady-state and transient kinetic data with in operando spectra provides a methodology to quantitatively describe the rate dependences of SCR reduction and oxidation processes on Cu-zeolite properties such as Cu ion density as shown here, and others including the zeolite framework topology and its density and distribution of Al atoms.
中文翻译:

对NO的速率双氧压力的影响X选择性催化还原用NH 3上的Cu-CHA沸石
在低温(<523 K)下,通过NH 3溶剂化的Cu离子催化的基本步骤,在Cu交换的沸石上用NH 3选择性催化还原NO(SCR),这是Cu II / Cu I中的反应性中间体氧化还原循环。SCR速率通常是在“标准”反应条件下测量的,其中O 2是氧化剂,并以分压(〜10 kPa O 2)出现,导致单点Cu II还原和双点Cu I还原氧化作用表现为动力学上相关的步骤。结果,随着铜含量的增加,空间密度(Si / Al)的增加,“标准” SCR速率(每克)从二阶到一阶依赖于孤立的铜含量(每克)。 = 15,Cu / Al = 0.08-0.37),因为双位I Cu I氧化步骤将SCR速率限制在较小范围内。在给定的Cu-CHA催化剂上,SCR速率(每Cu,473 K)在大范围变化(0–60 kPa O 2)时显示出朗缪尔对双氧压力的依赖性,从而能够分离出一级或零级动力学关于O 2。这些动力学机制分别对应于Cu I氧化或Cu II的极限条件还原成为支配动力学相关步骤,具有一致的在operando X射线吸收光谱。一阶速率常数(每Cu)随Cu密度近似线性增加,反映了O 2辅助Cu I氧化步骤的双重作用。零级速率常数(每Cu)随Cu密度的增加而逐渐增加,部分反映了能够形成双核中间物并因此参与SCR转换的分离的Cu离子的比例增加。将稳态和瞬态动力学数据与in操作相结合 光谱图提供了一种定量描述SCR还原和氧化过程对Cu沸石性能(如此处所示的Cu离子密度)以及其他包括沸石骨架拓扑及其Al原子密度和分布的速率依赖性的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号