当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper(I)-catalyzed highly enantioselective [3 + 3]-cycloaddition of γ-alkyl enoldiazoacetates with nitrones
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-05-27 , DOI: 10.1039/d0qo00539h Kuiyong Dong 1, 2, 3, 4, 5 , Xinfang Xu 5, 6, 7, 8, 9 , Michael P. Doyle 1, 2, 3, 4
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-05-27 , DOI: 10.1039/d0qo00539h Kuiyong Dong 1, 2, 3, 4, 5 , Xinfang Xu 5, 6, 7, 8, 9 , Michael P. Doyle 1, 2, 3, 4
Affiliation
|
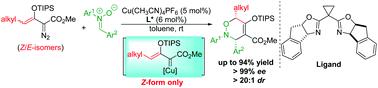
Chiral copper(I) catalysts are preferred over chiral dirhodium(II) catalysts for [3 + 3]-cycloaddition reactions of γ-alkyl-substituted enoldiazoacetates compounds with nitrones. Using the In-SaBox ligand these reactions effectively produce cis-3,6-dihydro-1,2-oxazine derivatives under mild conditions in high yield and with exceptional stereocontrol, and enantioselectivity increases with the size of the γ-substituent. Mechanistic studies show that cycloaddition occurs solely through the formation of (Z)-γ-substituted metallo-enolcarbene intermediates that are catalytically gennerated from both (Z)- and (E)-γ-substituted enoldiazoactates via donor–acceptor cyclopropene intermediates.
中文翻译:

铜(I)催化的高对映选择性[3 + 3]-γ-烷基烯氧基乙酸烷基酯与硝酮的环加成反应
对于γ-烷基取代的烯丙基氧乙酸酯化合物与硝酮的[3 + 3]-环加成反应而言,手性铜(I)催化剂优于手性铜(II)催化剂。使用In-SaBox配体,这些反应可在温和条件下以高收率和出色的立体控制有效地产生顺式-3,6-二氢-1,2-恶嗪衍生物,对映选择性随γ-取代基的大小而增加。机理的研究表明,通过环加成的(形成仅发生Ž)-γ -取代的被催化从两个(gennerated金属- enolcarbene中间体Ž) -和(Ë)-γ-取代enoldiazoactates经由 供体-受体环丙烯中间体。
更新日期:2020-06-30
中文翻译:

铜(I)催化的高对映选择性[3 + 3]-γ-烷基烯氧基乙酸烷基酯与硝酮的环加成反应
对于γ-烷基取代的烯丙基氧乙酸酯化合物与硝酮的[3 + 3]-环加成反应而言,手性铜(I)催化剂优于手性铜(II)催化剂。使用In-SaBox配体,这些反应可在温和条件下以高收率和出色的立体控制有效地产生顺式-3,6-二氢-1,2-恶嗪衍生物,对映选择性随γ-取代基的大小而增加。机理的研究表明,通过环加成的(形成仅发生Ž)-γ -取代的被催化从两个(gennerated金属- enolcarbene中间体Ž) -和(Ë)-γ-取代enoldiazoactates经由 供体-受体环丙烯中间体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号