当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
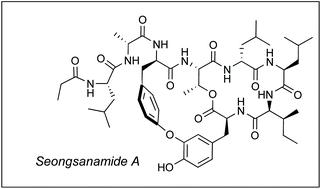
Total synthesis of antiallergic bicyclic peptide seongsanamide A
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-05-26 , DOI: 10.1039/d0qo00531b Feipeng Han 1, 2, 3 , Yian Guo 1, 2, 3 , Tao Ye 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-05-26 , DOI: 10.1039/d0qo00531b Feipeng Han 1, 2, 3 , Yian Guo 1, 2, 3 , Tao Ye 1, 2, 3, 4, 5
Affiliation
|
The first total synthesis of antiallergic depsipeptide seongsanamide A has been achieved and also the relative and absolute stereochemistry of the natural product has been confirmed. Highlights of the convergent route include the use of Miyuara borylation, Chan–Evans–Lam coupling for the effective assembly of the isodityrosine subunit and the identification of an effective macrocyclization site in very high conversion. The longest linear sequence leading to seongsanamide A was 12 steps, with an overall yield of 12.7%.
中文翻译:

抗过敏双环肽城山酰胺A的全合成
已经实现了抗过敏性二肽肽Seongsanamide A的第一个全合成,并且已经确认了天然产物的相对和绝对立体化学。融合路线的亮点包括使用Miyuara硼酸酯化,Chan–Evans–Lam偶联有效地组装异麦芽碱亚基,并确定高转化率的有效大环化位点。导致城山酰胺A的最长线性序列为12个步骤,总产率为12.7%。
更新日期:2020-06-30
中文翻译:

抗过敏双环肽城山酰胺A的全合成
已经实现了抗过敏性二肽肽Seongsanamide A的第一个全合成,并且已经确认了天然产物的相对和绝对立体化学。融合路线的亮点包括使用Miyuara硼酸酯化,Chan–Evans–Lam偶联有效地组装异麦芽碱亚基,并确定高转化率的有效大环化位点。导致城山酰胺A的最长线性序列为12个步骤,总产率为12.7%。



























 京公网安备 11010802027423号
京公网安备 11010802027423号