当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of GNE-149 as a Full Antagonist and Efficient Degrader of Estrogen Receptor alpha for ER+ Breast Cancer.
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2020-05-26 , DOI: 10.1021/acsmedchemlett.0c00224 Jun Liang 1 , Robert Blake 1 , Jae Chang 1 , Lori S Friedman 1 , Simon Goodacre 2 , Steven Hartman 1 , Ellen Rei Ingalla 1 , James R Kiefer 1 , Tracy Kleinheinz 1 , Sharada Labadie 1 , Jun Li 1 , Kwong Wah Lai 3 , Jiangpeng Liao 3 , Vidhi Mody 1 , Neville McLean 2 , Ciara Metcalfe 1 , Michelle Nannini 1 , Daniel Otwine 1 , Yingqing Ran 1 , Nick Ray 2 , Fabien Roussel 2 , Amy Sambrone 1 , Deepak Sampath 1 , Maia Vinogradova 1 , John Wai 3 , Tao Wang 3 , Kuen Yeap 2 , Amy Young 1 , Jason Zbieg 1 , Birong Zhang 1 , Xiaoping Zheng 3 , Yu Zhong 1 , Xiaojing Wang 1
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2020-05-26 , DOI: 10.1021/acsmedchemlett.0c00224 Jun Liang 1 , Robert Blake 1 , Jae Chang 1 , Lori S Friedman 1 , Simon Goodacre 2 , Steven Hartman 1 , Ellen Rei Ingalla 1 , James R Kiefer 1 , Tracy Kleinheinz 1 , Sharada Labadie 1 , Jun Li 1 , Kwong Wah Lai 3 , Jiangpeng Liao 3 , Vidhi Mody 1 , Neville McLean 2 , Ciara Metcalfe 1 , Michelle Nannini 1 , Daniel Otwine 1 , Yingqing Ran 1 , Nick Ray 2 , Fabien Roussel 2 , Amy Sambrone 1 , Deepak Sampath 1 , Maia Vinogradova 1 , John Wai 3 , Tao Wang 3 , Kuen Yeap 2 , Amy Young 1 , Jason Zbieg 1 , Birong Zhang 1 , Xiaoping Zheng 3 , Yu Zhong 1 , Xiaojing Wang 1
Affiliation
|
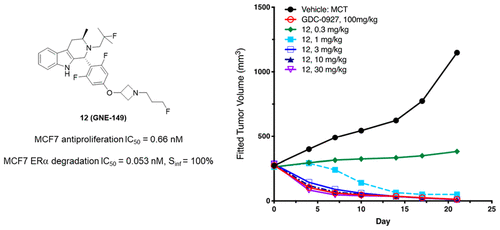
Estrogen receptor alpha (ERα) is a well-validated drug target for ER-positive (ER+) breast cancer. Fulvestrant is FDA-approved to treat ER+ breast cancer and works through two mechanisms—as a full antagonist and selective estrogen receptor degrader (SERD)—but lacks oral bioavailability. Thus, we envisioned a “best-in-class” molecule with the same dual mechanisms as fulvestrant, but with significant oral exposure. Through lead optimization, we discovered a tool molecule 12 (GNE-149) with improved degradation and antiproliferative activity in both MCF7 and T47D cells. To illustrate the binding mode and key interactions of this scaffold with ERα, we obtained a cocrystal structure of 6 that showed ionic interaction of azetidine with Asp351 residue. Importantly, 12 showed favorable metabolic stability and good oral exposure. 12 exhibited antagonist effect in the uterus and demonstrated robust dose-dependent efficacy in xenograft models.
中文翻译:

发现 GNE-149 作为 ER+ 乳腺癌雌激素受体 α 的完全拮抗剂和有效降解剂。
雌激素受体 α (ERα) 是经过充分验证的 ER 阳性 (ER+) 乳腺癌药物靶点。氟维司群经 FDA 批准用于治疗 ER+ 乳腺癌,通过两种机制发挥作用——作为完全拮抗剂和选择性雌激素受体降解剂 (SERD)——但缺乏口服生物利用度。因此,我们设想了一种“同类最佳”分子,具有与氟维司群相同的双重机制,但口服暴露量显着。通过先导化合物优化,我们发现了一种工具分子12 (GNE-149),在 MCF7 和 T47D 细胞中具有改善的降解和抗增殖活性。为了说明该支架与 ERα 的结合模式和关键相互作用,我们获得了6的共晶结构,该结构显示氮杂环丁烷与 Asp351 残基的离子相互作用。重要的是, 12显示出良好的代谢稳定性和良好的口服暴露。 12在子宫中表现出拮抗作用,并在异种移植模型中表现出强大的剂量依赖性功效。
更新日期:2020-05-26
中文翻译:

发现 GNE-149 作为 ER+ 乳腺癌雌激素受体 α 的完全拮抗剂和有效降解剂。
雌激素受体 α (ERα) 是经过充分验证的 ER 阳性 (ER+) 乳腺癌药物靶点。氟维司群经 FDA 批准用于治疗 ER+ 乳腺癌,通过两种机制发挥作用——作为完全拮抗剂和选择性雌激素受体降解剂 (SERD)——但缺乏口服生物利用度。因此,我们设想了一种“同类最佳”分子,具有与氟维司群相同的双重机制,但口服暴露量显着。通过先导化合物优化,我们发现了一种工具分子12 (GNE-149),在 MCF7 和 T47D 细胞中具有改善的降解和抗增殖活性。为了说明该支架与 ERα 的结合模式和关键相互作用,我们获得了6的共晶结构,该结构显示氮杂环丁烷与 Asp351 残基的离子相互作用。重要的是, 12显示出良好的代谢稳定性和良好的口服暴露。 12在子宫中表现出拮抗作用,并在异种移植模型中表现出强大的剂量依赖性功效。











































 京公网安备 11010802027423号
京公网安备 11010802027423号