当前位置:
X-MOL 学术
›
Plasma Processes Polym.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dielectric barrier discharge plasma treatment affects stability, metal ion coordination, and enzyme activity of bacterial superoxide dismutases
Plasma Processes and Polymers ( IF 2.9 ) Pub Date : 2020-05-27 , DOI: 10.1002/ppap.202000019 Marco Krewing 1 , Christoph K. Jung 2 , Elena Dobbelstein 1 , Britta Schubert 1 , Timo Jacob 2 , Julia E. Bandow 1
Plasma Processes and Polymers ( IF 2.9 ) Pub Date : 2020-05-27 , DOI: 10.1002/ppap.202000019 Marco Krewing 1 , Christoph K. Jung 2 , Elena Dobbelstein 1 , Britta Schubert 1 , Timo Jacob 2 , Julia E. Bandow 1
Affiliation
|
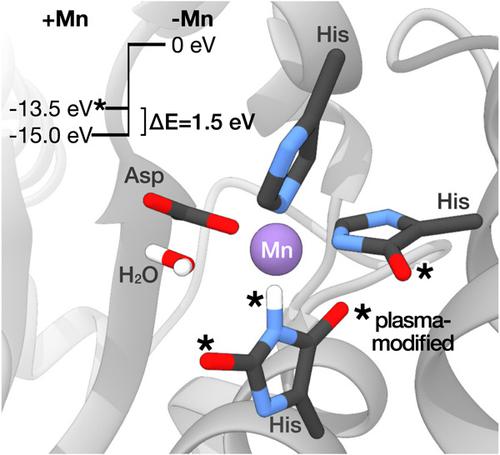
A molecular‐level understanding of the effects of atmospheric‐pressure plasma on biological samples requires knowledge of the effects on proteins. Superoxide dismutases, which detoxify superoxide under oxidative stress conditions, play a key role in bacterial plasma resistance. Investigation of the impact of dielectric barrier discharge (DBD) treatment on purified superoxide dismutases SodA and SodB of Escherichia coli showed that DBD treatment caused a rapid protein degradation, with only 8% of protein remaining after 10 min. The affinity of SodA for the metal cofactor Mn2+ was reduced. Mass spectrometry, in conjunction with coupled‐cluster calculations, revealed that modifications of amino acid residues in the active site can explain the decreased metal affinity and a distortion of the coordination geometry responsible for the activity loss.
中文翻译:

介电势垒放电等离子体处理影响细菌超氧化物歧化酶的稳定性,金属离子配位和酶活性
要从分子水平了解大气压等离子体对生物样品的影响,需要了解对蛋白质的影响。超氧化物歧化酶在氧化应激条件下使超氧化物解毒,在细菌的血浆抗性中起关键作用。对介电势垒放电(DBD)处理对大肠杆菌中纯化的超氧化物歧化酶SodA和SodB的影响的研究表明,DBD处理导致蛋白质快速降解,在10分钟后仅剩余8%的蛋白质。SodA对金属辅因子Mn 2+的亲和力减少了。质谱结合偶联簇计算表明,活性位点氨基酸残基的修饰可以解释金属亲和力降低和配位几何体变形的原因,从而导致活性降低。
更新日期:2020-05-27
中文翻译:

介电势垒放电等离子体处理影响细菌超氧化物歧化酶的稳定性,金属离子配位和酶活性
要从分子水平了解大气压等离子体对生物样品的影响,需要了解对蛋白质的影响。超氧化物歧化酶在氧化应激条件下使超氧化物解毒,在细菌的血浆抗性中起关键作用。对介电势垒放电(DBD)处理对大肠杆菌中纯化的超氧化物歧化酶SodA和SodB的影响的研究表明,DBD处理导致蛋白质快速降解,在10分钟后仅剩余8%的蛋白质。SodA对金属辅因子Mn 2+的亲和力减少了。质谱结合偶联簇计算表明,活性位点氨基酸残基的修饰可以解释金属亲和力降低和配位几何体变形的原因,从而导致活性降低。











































 京公网安备 11010802027423号
京公网安备 11010802027423号