当前位置:
X-MOL 学术
›
J. Cell. Physiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
P53 and H3K4me2 activate N6-methylated LncPGCAT-1 to regulate primordial germ cell formation via MAPK signaling.
Journal of Cellular Physiology ( IF 4.5 ) Pub Date : 2020-05-27 , DOI: 10.1002/jcp.29805 Qisheng Zuo 1, 2 , Jing Jin 1, 2 , Kai Jin 1, 2 , Jing Zhou 1, 2 , Changhua Sun 1, 2 , Jiuzhou Song 3 , Guohong Chen 1, 2 , Yani Zhang 1, 2 , Bichun Li 1, 2
Journal of Cellular Physiology ( IF 4.5 ) Pub Date : 2020-05-27 , DOI: 10.1002/jcp.29805 Qisheng Zuo 1, 2 , Jing Jin 1, 2 , Kai Jin 1, 2 , Jing Zhou 1, 2 , Changhua Sun 1, 2 , Jiuzhou Song 3 , Guohong Chen 1, 2 , Yani Zhang 1, 2 , Bichun Li 1, 2
Affiliation
|
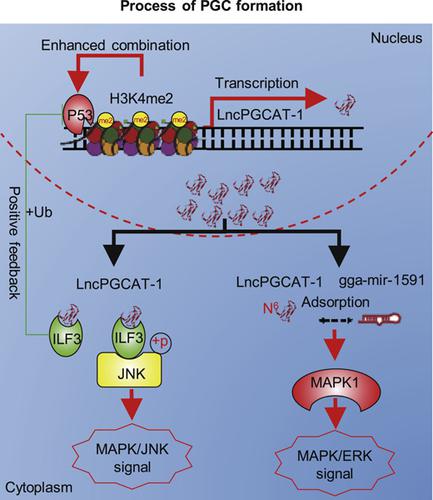
Long noncoding RNAs (lncRNAs) participate in the formation of primordial germ cells (PGCs); however, the identity of the key lncRNAs and the molecular mechanisms responsible for the formation of PGCs remain unknown. Here, we identify a key candidate lncRNA (lncRNA PGC transcript‐1, LncPGCAT‐1) via RNA sequencing of embryonic stem cells, PGCs, and Spermatogonial stem cells (SSCs). Functional experiments confirmed that LncPGCAT‐1 positively regulated the formation of PGCs by elevating the expression of Cvh and C‐kit while downregulating the pluripotency(Nanog) in vitro and in vivo; PAS staining of genital ridges in vivo also showed that interference with LncPGCAT‐1 can significantly reduce the number of PGCs in genital ridges, while overexpression of LncPGCAT‐1 had the opposite result. The result of luciferase reporter assay combined with CHIP‐qPCR showed that the expression of LncPGCAT‐1 was promoted by the transcription factor P53 and high levels of H3K4me2. Mechanistically, the luciferase reporter assay confirmed that mitogen‐activated protein kinase 1 (MAPK1) was the target gene of LncPGCAT‐1 and gga‐mir‐1591. In the ceRNA system, high levels of N6 methylation of LncPGCAT‐1 enhanced the adsorption capacity of LncPGCAT‐1 for gga‐mir‐1591. Adsorption of gga‐mir‐1591 activated the MAPK1/ERK signaling cascade by relieving the gga‐mir‐1591‐dependent inhibition of MAPK1 expression. Moreover, LncPGCAT‐1 interacted with interleukin enhancer binding factor 3 (ILF3) to regulate the ubiquitination of P53 and phosphorylation of JNK. Interaction with ILF3 resulted in positive self‐feedback regulation of LncPGCAT‐1 and activation of JNK signaling, ultimately promoting PGC formation. Altogether, the study expands our knowledge of the function and molecular mechanisms of lncRNAs in PGC development.
中文翻译:

P53和H3K4me2激活N6-甲基化的LncPGCAT-1,以通过MAPK信号传导调节原始生殖细胞的形成。
长非编码RNA(lncRNA)参与原始生殖细胞(PGC)的形成;然而,关键lncRNA的身份和负责PGC形成的分子机制仍然未知。在这里,我们通过对胚胎干细胞,PGC和精原干细胞(SSC)进行RNA测序,确定了关键候选lncRNA(lncRNA PGC transcript-1,LncPGCAT-1)。功能实验证实,LncPGCAT-1通过提高Cvh和C-kit的表达而在体外和体内下调多能性(Nanog),从而积极调节PGC的形成。体内生殖器s的PAS染色还显示对LncPGCAT-1的干扰可以显着减少生殖器中PGC的数量,而LncPGCAT-1的过表达则相反。萤光素酶报告基因检测结合CHIP-qPCR的结果表明,转录因子P53和高水平的H3K4me2促进LncPGCAT-1的表达。从机械上讲,荧光素酶报告基因测定证实了促分裂原激活的蛋白激酶1(MAPK1)是LncPGCAT-1和gga-mir-1591的靶基因。在ceRNA系统中,LncPGCAT-1的高水平N 6甲基化增强了LncPGCAT-1对gga-mir- 1159的吸附能力。gga-mir-1591的吸附可通过减轻gga-mir-1591依赖性的MAPK1表达抑制来激活MAPK1 / ERK信号级联。此外,LncPGCAT-1与白介素增强子结合因子3(ILF3)相互作用,调节P53的泛素化和JNK的磷酸化。与ILF3的相互作用导致LncPGCAT-1的正自我反馈调节和JNK信号的激活,最终促进PGC的形成。总而言之,这项研究扩展了我们对lncRNA在PGC发育中的功能和分子机制的认识。
更新日期:2020-05-27
中文翻译:

P53和H3K4me2激活N6-甲基化的LncPGCAT-1,以通过MAPK信号传导调节原始生殖细胞的形成。
长非编码RNA(lncRNA)参与原始生殖细胞(PGC)的形成;然而,关键lncRNA的身份和负责PGC形成的分子机制仍然未知。在这里,我们通过对胚胎干细胞,PGC和精原干细胞(SSC)进行RNA测序,确定了关键候选lncRNA(lncRNA PGC transcript-1,LncPGCAT-1)。功能实验证实,LncPGCAT-1通过提高Cvh和C-kit的表达而在体外和体内下调多能性(Nanog),从而积极调节PGC的形成。体内生殖器s的PAS染色还显示对LncPGCAT-1的干扰可以显着减少生殖器中PGC的数量,而LncPGCAT-1的过表达则相反。萤光素酶报告基因检测结合CHIP-qPCR的结果表明,转录因子P53和高水平的H3K4me2促进LncPGCAT-1的表达。从机械上讲,荧光素酶报告基因测定证实了促分裂原激活的蛋白激酶1(MAPK1)是LncPGCAT-1和gga-mir-1591的靶基因。在ceRNA系统中,LncPGCAT-1的高水平N 6甲基化增强了LncPGCAT-1对gga-mir- 1159的吸附能力。gga-mir-1591的吸附可通过减轻gga-mir-1591依赖性的MAPK1表达抑制来激活MAPK1 / ERK信号级联。此外,LncPGCAT-1与白介素增强子结合因子3(ILF3)相互作用,调节P53的泛素化和JNK的磷酸化。与ILF3的相互作用导致LncPGCAT-1的正自我反馈调节和JNK信号的激活,最终促进PGC的形成。总而言之,这项研究扩展了我们对lncRNA在PGC发育中的功能和分子机制的认识。











































 京公网安备 11010802027423号
京公网安备 11010802027423号