当前位置:
X-MOL 学术
›
Energy Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper Selenide–Derived Copper Oxide Nanoplates as a Durable and Efficient Electrocatalyst for Oxygen Evolution Reaction
Energy Technology ( IF 3.6 ) Pub Date : 2020-05-27 , DOI: 10.1002/ente.202000142 Xiaoxiao Wang 1 , Xiyan Hou 2 , Husileng Lee 1 , Liangjie Lu 1 , Xiujuan Wu 1 , Licheng Sun 1, 3, 4
Energy Technology ( IF 3.6 ) Pub Date : 2020-05-27 , DOI: 10.1002/ente.202000142 Xiaoxiao Wang 1 , Xiyan Hou 2 , Husileng Lee 1 , Liangjie Lu 1 , Xiujuan Wu 1 , Licheng Sun 1, 3, 4
Affiliation
|
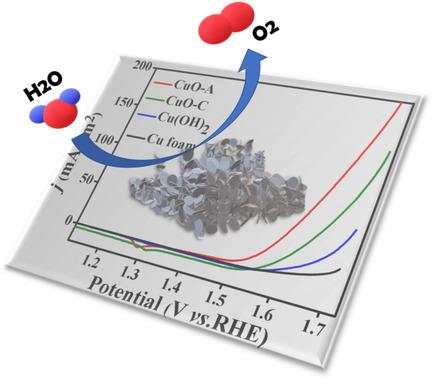
Earth‐abundant transition metal–based nanomaterials play a significant role in oxygen evolution reaction (OER). Among them, copper has attracted significant attention due to its excellent electrocatalytic activity, low price, and abundance. Herein, a nanostructured copper oxide (CuO‐A) is generated in situ from a cuprous selenide (Cu2Se) precursor under oxygen evolution reaction conditions. The as‐prepared CuO‐A/copper foam (CF) electrode delivers a current density of 10 mA cm−2 at an overpotential of 297 mV with good stability for over 50 h in 1 m KOH solution, which is superior to most recently reported copper‐based water oxidation catalysts. The high catalytic performance of CuO‐A is mainly attributed to the improved surface area offered by the morphology reconstruction during the in situ transformation process. As a result, it paves a way to synthesize effective and stable transition metal oxide catalysts via the in situ conversion of transition metal chalcogenides for energy conversion and storage applications.
中文翻译:

硒化铜衍生的氧化铜纳米板可作为一种持久高效的氧析出反应电催化剂
富含地球的过渡金属基纳米材料在氧释放反应(OER)中起着重要作用。其中,铜因其出色的电催化活性,低廉的价格和丰富的性能而引起了人们的极大关注。在此,在氧放出反应条件下,从硒化亚铜(Cu 2 Se)前驱体原位生成了纳米结构的氧化铜(CuO-A)。所制备的CuO-A /铜泡沫(CF)电极在297 mV的过电势下可提供10 mA cm -2的电流密度,并在1 m的50小时内具有良好的稳定性 KOH溶液,优于最近报道的铜基水氧化催化剂。CuO-A的高催化性能主要归因于原位转化过程中形态重构带来的表面积增加。结果,它为通过过渡金属硫族化物的原位转化为能量转化和存储应用提供了一种有效而稳定的过渡金属氧化物催化剂的合成方法。
更新日期:2020-07-02
中文翻译:

硒化铜衍生的氧化铜纳米板可作为一种持久高效的氧析出反应电催化剂
富含地球的过渡金属基纳米材料在氧释放反应(OER)中起着重要作用。其中,铜因其出色的电催化活性,低廉的价格和丰富的性能而引起了人们的极大关注。在此,在氧放出反应条件下,从硒化亚铜(Cu 2 Se)前驱体原位生成了纳米结构的氧化铜(CuO-A)。所制备的CuO-A /铜泡沫(CF)电极在297 mV的过电势下可提供10 mA cm -2的电流密度,并在1 m的50小时内具有良好的稳定性 KOH溶液,优于最近报道的铜基水氧化催化剂。CuO-A的高催化性能主要归因于原位转化过程中形态重构带来的表面积增加。结果,它为通过过渡金属硫族化物的原位转化为能量转化和存储应用提供了一种有效而稳定的过渡金属氧化物催化剂的合成方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号