当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Small-Molecule Inhibitors Targeting Sterol 14α-Demethylase (CYP51): Synthesis, Molecular Modelling and Evaluation Against Candida albicans.
ChemMedChem ( IF 3.6 ) Pub Date : 2020-05-27 , DOI: 10.1002/cmdc.202000250 Faizah A Binjubair 1 , Josie E Parker 2 , Andrew G Warrilow 2 , Kalika Puri 1 , Peter J Braidley 1 , Esra Tatar 1, 3 , Steven L Kelly 2 , Diane E Kelly 2 , Claire Simons 1
ChemMedChem ( IF 3.6 ) Pub Date : 2020-05-27 , DOI: 10.1002/cmdc.202000250 Faizah A Binjubair 1 , Josie E Parker 2 , Andrew G Warrilow 2 , Kalika Puri 1 , Peter J Braidley 1 , Esra Tatar 1, 3 , Steven L Kelly 2 , Diane E Kelly 2 , Claire Simons 1
Affiliation
|
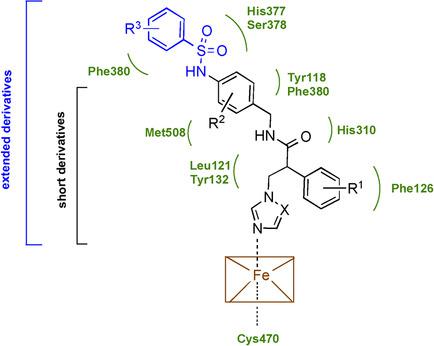
Fungal infections are a global issue affecting over 150 million people worldwide annually, with 750 000 of these caused by invasive Candida infections. Azole drugs are the frontline treatment against fungal infections; however, resistance to current azole antifungals in C. albicans poses a threat to public health. Two series of novel azole derivatives, short and extended derivatives, have been designed, synthesised and investigated for CYP51 inhibitory activity, binding affinity and minimum inhibitory concentration (MIC) against C. albicans strains. The short derivatives were more potent against the C. albicans strains (e. g., MIC 2‐(4‐chlorophenyl)‐N‐(2,4‐dichlorobenzyl)‐3‐(1H‐imidazol‐1‐yl)propanamide (5 f ) <0.03 μg/mL, N‐(4‐((4‐chlorophenyl)sulfonamido)benzyl)‐2‐phenyl‐3‐(1H‐1,2,4‐triazol‐1‐yl)propanamide (12 c ), 1 μg/mL, fluconazole 0.125 μg/mL) but both displayed comparable enzyme binding and inhibition (5 f K d 62±17 nM, IC50 0.46 μM; 12 c K d 43±18 nM, IC50 0.33 μM, fluconazole K d 41±13 nM, IC50 0.31 μM, posaconazole K d 43±11 nM, IC50 0.2 μM). The short series had poor selectivity for CaCYP51 over the human homologue, whereas the selectivity of the extended series, for example, compound 12 c , was higher (21.5‐fold) than posaconazole (4.7‐fold) based on K d values, although posaconazole was more selective (615‐fold) than 12 c (461‐fold) based on IC50 values. Based on inhibitory activity and selectivity profile, the extended series are the better of the two series for further development.
中文翻译:

针对甾醇 14α-脱甲基酶 (CYP51) 的小分子抑制剂:针对白色念珠菌的合成、分子建模和评估。
真菌感染是一个全球性问题,每年影响全球超过 1.5 亿人,其中 75 万人是由侵入性念珠菌感染引起的。唑类药物是治疗真菌感染的一线药物;然而,白色念珠菌对现有唑类抗真菌药物的耐药性对公众健康构成威胁。设计、合成了两个系列的新型唑类衍生物,即短衍生物和延长衍生物,并研究了其对白色念珠菌菌株的 CYP51 抑制活性、结合亲和力和最低抑制浓度 (MIC)。短衍生物对白色念珠菌菌株更有效(例如,MIC 2-(4-氯苯基) -N- (2,4-二氯苄基)-3-(1 H-咪唑-1-基)丙酰胺 ( 5 f )<0.03 μg/mL, N- (4-((4-氯苯基)磺酰胺基)苄基)-2-苯基-3-(1 H -1,2,4-三唑-1-基)丙酰胺 ( 12 c ),1 μg/mL,氟康唑 0.125 μg/mL),但两者都显示出相当的酶结合和抑制作用( 5 f K d 62±17 nM,IC 50 0.46 μM; 12 c K d 43±18 nM,IC 50 0.33 μM ,氟康唑K d 41±13 nM,IC 50 0.31 μM,泊沙康唑K d 43±11 nM,IC 50 0.2 μM)。与人类同系物相比,短系列对 CaCYP51 的选择性较差,而扩展系列(例如化合物12 c )的选择性高于泊沙康唑(4.5 倍)(21.5 倍)。根据K d值,泊沙康唑比12 c (461 倍)更具选择性(615 倍),但根据 IC 50值,泊沙康唑的选择性更高(615 倍)。根据抑制活性和选择性特征,扩展系列是两个系列中更好的一个,有利于进一步开发。
更新日期:2020-07-20
中文翻译:

针对甾醇 14α-脱甲基酶 (CYP51) 的小分子抑制剂:针对白色念珠菌的合成、分子建模和评估。
真菌感染是一个全球性问题,每年影响全球超过 1.5 亿人,其中 75 万人是由侵入性念珠菌感染引起的。唑类药物是治疗真菌感染的一线药物;然而,白色念珠菌对现有唑类抗真菌药物的耐药性对公众健康构成威胁。设计、合成了两个系列的新型唑类衍生物,即短衍生物和延长衍生物,并研究了其对白色念珠菌菌株的 CYP51 抑制活性、结合亲和力和最低抑制浓度 (MIC)。短衍生物对白色念珠菌菌株更有效(例如,MIC 2-(4-氯苯基) -N- (2,4-二氯苄基)-3-(1 H-咪唑-1-基)丙酰胺 ( 5 f )<0.03 μg/mL, N- (4-((4-氯苯基)磺酰胺基)苄基)-2-苯基-3-(1 H -1,2,4-三唑-1-基)丙酰胺 ( 12 c ),1 μg/mL,氟康唑 0.125 μg/mL),但两者都显示出相当的酶结合和抑制作用( 5 f K d 62±17 nM,IC 50 0.46 μM; 12 c K d 43±18 nM,IC 50 0.33 μM ,氟康唑K d 41±13 nM,IC 50 0.31 μM,泊沙康唑K d 43±11 nM,IC 50 0.2 μM)。与人类同系物相比,短系列对 CaCYP51 的选择性较差,而扩展系列(例如化合物12 c )的选择性高于泊沙康唑(4.5 倍)(21.5 倍)。根据K d值,泊沙康唑比12 c (461 倍)更具选择性(615 倍),但根据 IC 50值,泊沙康唑的选择性更高(615 倍)。根据抑制活性和选择性特征,扩展系列是两个系列中更好的一个,有利于进一步开发。











































 京公网安备 11010802027423号
京公网安备 11010802027423号