Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-05-27 , DOI: 10.1016/j.jmb.2020.05.017 Jiemin Shen 1 , Gang Wu 2 , Ah-Lim Tsai 2 , Ming Zhou 1
|
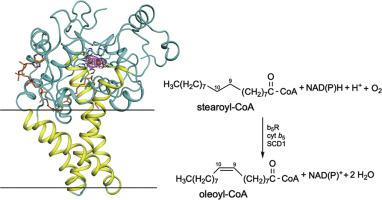
Stearoyl-CoA desaturase 1 (SCD1) is a membrane-embedded metalloenzyme that catalyzes the formation of a double bond on a saturated acyl-CoA. SCD1 has a diiron center and its proper function requires an electron transport chain composed of NADH (or NADPH), cytochrome b5 reductase (b5R), and cytochrome b5 (cyt b5). Since SCD1 is a key regulator in fat metabolism and is required for survival of cancer cells, there is intense interest in targeting SCD1 for various metabolic diseases and cancers. Crystal structures of human and mouse SCD1 were reported recently; however, both proteins have two zinc ions instead of two iron ions in the catalytic center, and as a result, the enzymes are inactive. Here we report a general approach for incorporating iron into heterologously expressed proteins in HEK293 cells. We produced mouse SCD1 that contains a diiron center and visualized its diiron center by solving its crystal structure to 3.5 Å. We assembled the entire electron transport chain using the purified soluble domains of cyt b5 and b5R, and the purified mouse SCD1, and we showed that three proteins coordinate to produce proper products. These results established an in vitro system that allows precise perturbations of the electron transport chain for the understanding of the catalytic mechanism in SCD1.
中文翻译:

哺乳动物硬脂酰辅酶A去饱和酶中独特的二铁中心的结构和机制。
硬脂酰辅酶 A 去饱和酶 1 (SCD1) 是一种膜嵌入金属酶,可催化饱和酰基辅酶 A 上双键的形成。 SCD1具有二铁中心,其正常功能需要由NADH(或NADPH)、细胞色素b 5还原酶(b 5 R)和细胞色素b 5 (cyt b 5 )组成的电子传递链。由于 SCD1 是脂肪代谢的关键调节因子并且是癌细胞生存所必需的,因此人们对靶向 SCD1 治疗各种代谢疾病和癌症产生了浓厚的兴趣。最近报道了人和小鼠SCD1的晶体结构;然而,这两种蛋白质的催化中心都有两个锌离子而不是两个铁离子,因此酶没有活性。在这里,我们报告了一种将铁掺入 HEK293 细胞中异源表达蛋白质的通用方法。我们生产了含有二铁中心的小鼠 SCD1,并通过将其晶体结构解析为 3.5 Å 来可视化其二铁中心。我们使用纯化的 cyt b 5和 b 5 R 的可溶性结构域以及纯化的小鼠 SCD1 组装了整个电子传递链,并且我们表明三种蛋白质协调产生适当的产物。这些结果建立了一个体外系统,可以精确扰动电子传输链,以了解 SCD1 的催化机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号