Journal of Catalysis ( IF 6.5 ) Pub Date : 2020-05-26 , DOI: 10.1016/j.jcat.2020.05.021 Vibin Vargheese , Junichi Murakami , Kyoko K. Bando , I. Tyrone Ghampson , Gwang-Nam Yun , Yasukazu Kobayashi , S. Ted Oyama
|
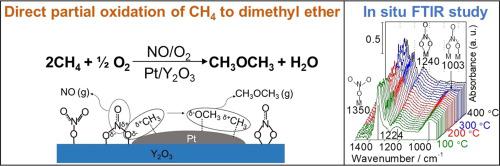
The direct partial oxidation of CH4 to dimethyl ether (DME) on a Pt/Y2O3 catalyst was studied using a mixture of NO and O2 as the oxidant. The reaction was carried out in a fixed bed reactor at 0.1 MPa and 275–400 °C using 20% CH4, 1% NO, and 1% O2 in inert gas. No methanol was detected in the effluent and a contact time study demonstrated that DME was a primary product. The DME productivities were comparable to the oxygenate (methanol, formaldehyde) productivities obtained with stronger oxidants such as N2O, H2O2, and O3. The presence of Pt and the NO + O2 gas mixture was necessary for DME formation; without NO only CO2 was produced. During the methane partial oxidation reaction NO and NO2 were not reduced to N2, indicating that they worked as a shuttle to transfer oxygen from O2 to CH4. In situ Fourier transform infrared showed the formation of a bridged nitrate species on the Pt/Y2O3 catalyst which was associated with the reaction of CH4. A comprehensive study of this bridged nitrate species indicated they were formed on yttria sites close to Pt and were likely responsible for the formation of DME. The characterization of catalysts using X-ray diffraction showed that the Pt was highly dispersed and CO uptake measurements indicated a particle size of ~3 nm. Analysis by X-ray absorption fine structure measurements showed the presence of Pt oxide with Pt-O and Pt-Pt bonds.
中文翻译:

使用NO / NO 2氧原子穿梭在Pt / Y 2 O 3催化剂上将CH 4直接分子氧部分氧化为二甲醚而不会形成甲醇
使用NO和O 2的混合物作为氧化剂,研究了在Pt / Y 2 O 3催化剂上CH 4直接部分氧化为二甲醚(DME)。该反应是在固定床反应器中于0.1 MPa和275–400°C下使用惰性气体中的20%CH 4,1%NO和1%O 2进行的。在废水中未检测到甲醇,接触时间研究表明DME是主要产物。DME生产率可与使用强氧化剂(例如N 2 O,H 2 O 2和O 3)获得的含氧化合物(甲醇,甲醛)生产率相媲美。Pt和NO + O 2的存在形成DME所需的气体混合物; 没有NO,仅产生CO 2。在甲烷部分氧化反应过程中,NO和NO 2未被还原为N 2,表明它们可作为将氧气从O 2转移至CH 4的梭子。原位傅立叶变换红外光谱显示在Pt / Y 2 O 3催化剂上形成桥连的硝酸盐类物质,这与CH 4的反应有关。对这种桥接的硝酸盐物种的全面研究表明,它们是在靠近Pt的氧化钇位点上形成的,可能是DME形成的原因。使用X射线衍射对催化剂进行的表征表明,Pt高度分散,CO吸收测量表明粒径约为3 nm。通过X射线吸收精细结构测量的分析表明存在具有Pt-O和Pt-Pt键的Pt氧化物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号