当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Radical addition-polar termination cascade: efficient strategy for photoredox-neutral-catalysed cyclopropanation and Giese-type reactions of alkenyl N-methyliminodiacetyl boronates
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-05-26 , DOI: 10.1039/d0qo00349b Yongjun Liu 1, 2, 3, 4 , Wenping Luo 1, 2, 3, 4 , Jiali Wu 4, 5, 6, 7 , Yewen Fang 4, 5, 6, 7 , Yan Li 1, 2, 3, 4 , Xiaoping Jin 4, 8, 9, 10 , Li Zhang 4, 8, 9, 10 , Zongyong Zhang 4, 5, 6, 7 , Fenfen Xu 4, 5, 6, 7 , Chan Du 4, 5, 6, 7
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-05-26 , DOI: 10.1039/d0qo00349b Yongjun Liu 1, 2, 3, 4 , Wenping Luo 1, 2, 3, 4 , Jiali Wu 4, 5, 6, 7 , Yewen Fang 4, 5, 6, 7 , Yan Li 1, 2, 3, 4 , Xiaoping Jin 4, 8, 9, 10 , Li Zhang 4, 8, 9, 10 , Zongyong Zhang 4, 5, 6, 7 , Fenfen Xu 4, 5, 6, 7 , Chan Du 4, 5, 6, 7
Affiliation
|
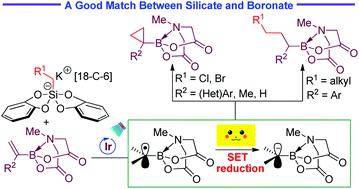
With radical addition-polar termination (RAPT) cascade as the strategy, efficient cyclopropanation and Giese-type reactions of alkenyl N-methyliminodiacetyl boronates have been realized by means of photoredox catalysis. With chloro- or bromomethyl silicate as the methylene source, a variety of cyclopropyl MIDA boronates as air- and chromatographically stable solids were prepared via photoredox-catalysed cyclopropanation of α-MIDA-boryl styrenes, 2-propenyl-BMIDA, and ethenyl-BMIDA. Moreover, photocatalytic Giese-type reactions of alkyl radicals with α-MIDA-boryl styrenes were also developed. It is essential for the successful radical conjugate addition employing alkyl silicates as the radical precursor. These RAPT cascades are characterized by a redox-neutral and additive-free process.
中文翻译:

自由基加成-极性终止级联:烯基N-甲基亚氨基二乙酰基硼酸酯的光氧化还原中性催化环丙烷化和Giese型反应的有效策略
以自由基加成-极性终止(RAPT)级联为策略,已经通过光氧化还原催化实现了烯基N-甲基亚氨基二乙酰基硼酸酯的有效环丙烷化和Giese型反应。以氯或溴代甲基丙烯酸硅作为亚甲基源,通过以下方法制备了多种环丙基MIDA硼酸酯,它们是空气和色谱稳定的固体光氧化还原催化的α-MIDA-硼苯乙烯,2-丙烯基-BMIDA和乙烯基-BMIDA的环丙烷化。此外,还开发了烷基与α-MIDA-硼苯乙烯的光催化吉斯型反应。对于使用烷基硅酸盐作为自由基前体的成功的自由基共轭加成,这是必不可少的。这些RAPT级联的特征是氧化还原中性且无添加剂。
更新日期:2020-06-30
中文翻译:

自由基加成-极性终止级联:烯基N-甲基亚氨基二乙酰基硼酸酯的光氧化还原中性催化环丙烷化和Giese型反应的有效策略
以自由基加成-极性终止(RAPT)级联为策略,已经通过光氧化还原催化实现了烯基N-甲基亚氨基二乙酰基硼酸酯的有效环丙烷化和Giese型反应。以氯或溴代甲基丙烯酸硅作为亚甲基源,通过以下方法制备了多种环丙基MIDA硼酸酯,它们是空气和色谱稳定的固体光氧化还原催化的α-MIDA-硼苯乙烯,2-丙烯基-BMIDA和乙烯基-BMIDA的环丙烷化。此外,还开发了烷基与α-MIDA-硼苯乙烯的光催化吉斯型反应。对于使用烷基硅酸盐作为自由基前体的成功的自由基共轭加成,这是必不可少的。这些RAPT级联的特征是氧化还原中性且无添加剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号