当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Revealing the Specificity of a Range of Antimicrobial Peptides in Lipid Nanodiscs by Native Mass Spectrometry.
Biochemistry ( IF 2.9 ) Pub Date : 2020-05-26 , DOI: 10.1021/acs.biochem.0c00335 Lawrence R. Walker , Michael T. Marty
Biochemistry ( IF 2.9 ) Pub Date : 2020-05-26 , DOI: 10.1021/acs.biochem.0c00335 Lawrence R. Walker , Michael T. Marty
|
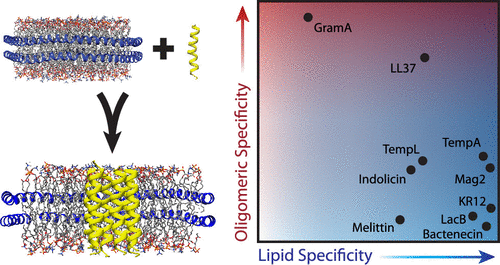
Antimicrobial peptides (AMPs) interact directly with lipid membranes of pathogens and may have the potential to combat antibiotic resistance. Although many AMPs are thought to form toxic oligomeric pores, their interactions within lipid membranes are not well understood. Here, we used native mass spectrometry to measure the incorporation of a range of different AMPs in lipoprotein nanodiscs. We found that the truncation of human LL37 increases the lipid specificity but decreases the specificity of complex formation. We also saw that the reduction of disulfide bonds can have a dramatic effect on the ability of AMPs to interact with lipid bilayers. Finally, by examining a wider range of peptides we discovered that AMPs tend to interact specifically with anionic lipids but form nonspecific complexes with wide oligomeric state distributions. Overall, these data reveal that each AMP has unique behaviors but some common trends apply to many AMPs.
中文翻译:

通过天然质谱揭示脂质纳米盘中一系列抗菌肽的特异性。
抗菌肽(AMP)与病原体的脂膜直接相互作用,可能具有抵抗抗生素耐药性的潜力。尽管许多AMP被认为会形成有毒的寡聚孔,但它们在脂质膜内的相互作用尚不十分清楚。在这里,我们使用天然质谱仪来测量脂蛋白纳米光盘中一系列不同AMP的掺入。我们发现人LL37的截断增加了脂质特异性,但降低了复合物形成的特异性。我们还看到,二硫键的还原可对AMP与脂质双层相互作用的能力产生重大影响。最后,通过检查更广泛的肽,我们发现AMP倾向于与阴离子脂质特异性相互作用,但形成具有宽寡聚态分布的非特异性复合物。总体,
更新日期:2020-05-26
中文翻译:

通过天然质谱揭示脂质纳米盘中一系列抗菌肽的特异性。
抗菌肽(AMP)与病原体的脂膜直接相互作用,可能具有抵抗抗生素耐药性的潜力。尽管许多AMP被认为会形成有毒的寡聚孔,但它们在脂质膜内的相互作用尚不十分清楚。在这里,我们使用天然质谱仪来测量脂蛋白纳米光盘中一系列不同AMP的掺入。我们发现人LL37的截断增加了脂质特异性,但降低了复合物形成的特异性。我们还看到,二硫键的还原可对AMP与脂质双层相互作用的能力产生重大影响。最后,通过检查更广泛的肽,我们发现AMP倾向于与阴离子脂质特异性相互作用,但形成具有宽寡聚态分布的非特异性复合物。总体,



























 京公网安备 11010802027423号
京公网安备 11010802027423号