Journal of Water Process Engineering ( IF 6.3 ) Pub Date : 2020-05-26 , DOI: 10.1016/j.jwpe.2020.101314 Arvind Kumar , Basheswar Prasad , Krishan Kishor Garg
|
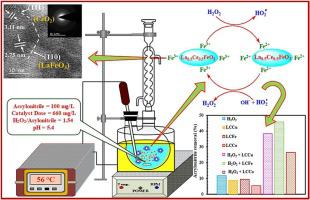
The present study focuses on catalytic degradation of carcinogenic and refractory organic compound (acrylonitrile) with La0.5Ce0.5MO3 (M = Fe, Cu and Co) perovskite-like catalysts synthesized by sol-gel method were examined by catalytic peroxidation process. Various analytical techniques, including XRD, FTIR, BET surface area, XPS, FE-SEM with EDAX and TEM were used for catalyst characterization. During catalytic peroxidation, influence of various operating parameters such as, catalyst dose (250–1250 mg/L), H2O2/ acrylonitrile (0.5–2.5), pH (2–10) and reaction temperature (30–90 °C) was studied and optimized through central composite design (CCD) in response surface methodology (RSM). Maximum acrylonitrile removal of 90.11% was observed at optimum operating conditions (i.e., LCFe catalyst dose 660 mg/L, H2O2/ acrylonitrile molar ratio 1.54, pH 5.4, reaction temperature 56.7 °C and reaction time 3 h). Two-step first order kinetics was performed at optimum operating conditions for the degradation of acrylonitrile at various temperatures and concentrations. To assess the economic viability of catalyst, reusability study was executed over four cycles of experiments at optimum operating conditions. The acrylonitrile removal was gradually dropped from 90.11% to 80.38%, 73.42% and 69.92%, respectively in second, third and fourth cycles. The proposed pathways of acrylonitrile degradation were suggested on the basis of intermediates identified by GC–MS analysis and reactive oxidant species (ROS) study. The operating cost of acrylonitrile treatment from aqueous solution was estimated to be 61.14 $/m3 wastewater by catalytic peroxidation process.
中文翻译:

La 0.5 Ce 0.5 MO 3(M = Fe,Cu和Co)钙钛矿状催化剂可从水溶液中高效降解丙烯腈:优化和反应途径
本文研究了通过溶胶-凝胶法合成的La 0.5 Ce 0.5 MO 3(M = Fe,Cu和Co)钙钛矿状催化剂对致癌和难处理有机化合物(丙烯腈)的催化降解,并通过催化过氧化工艺进行了研究。使用各种分析技术,包括XRD,FTIR,BET表面积,XPS,具有EDAX的FE-SEM和TEM进行催化剂表征。在催化过氧化过程中,各种操作参数的影响,例如催化剂剂量(250-1250 mg / L),H 2 O 2/丙烯腈(0.5–2.5),pH(2–10)和反应温度(30–90°C)已通过响应表面方法(RSM)的中央复合设计(CCD)进行了研究和优化。在最佳操作条件下(例如,LCFe催化剂剂量为660 mg / L,H 2 O 2)观察到最大丙烯腈去除率为90.11%/丙烯腈的摩尔比为1.54,pH为5.4,反应温度为56.7℃,反应时间为3小时。在最佳操作条件下进行了两步一级动力学,以在各种温度和浓度下降解丙烯腈。为了评估催化剂的经济可行性,在最佳操作条件下进行了四个实验周期的可重复使用性研究。在第二,第三和第四次循环中,丙烯腈的去除率分别从90.11%降至80.38%,73.42%和69.92%。在通过GC-MS分析和活性氧化剂(ROS)研究确定的中间体的基础上,提出了丙烯腈降解的建议途径。由水溶液处理丙烯腈的运行成本估计为61.14 $ / m 3 废水通过催化过氧化过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号