Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-05-26 , DOI: 10.1016/j.jmb.2020.05.018 Yuan Gao 1 , Cong Guo 2 , Jens O Watzlawik 3 , Peter S Randolph 4 , Elizabeth J Lee 1 , Danting Huang 1 , Scott M Stagg 5 , Huan-Xiang Zhou 6 , Terrone L Rosenberry 3 , Anant K Paravastu 1
|
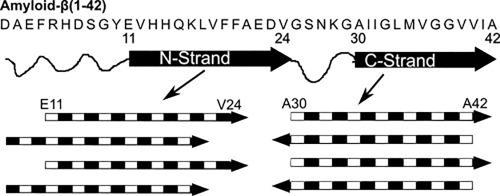
We present solid-state NMR measurements of β-strand secondary structure and inter-strand organization within a 150-kDa oligomeric aggregate of the 42-residue variant of the Alzheimer's amyloid-β peptide (Aβ(1–42)). We build upon our previous report of a β-strand spanned by residues 30–42, which arranges into an antiparallel β-sheet. New results presented here indicate that there is a second β-strand formed by residues 11–24. Contrary to expectations, NMR data indicate that this second β-strand is organized into a parallel β-sheet despite the co-existence of an antiparallel β-sheet in the same structure. In addition, the in-register parallel β-sheet commonly observed for amyloid fibril structure does not apply to residues 11–24 in the 150-kDa oligomer. Rather, we present evidence for an inter-strand registry shift of three residues that likely alternate in direction between adjacent molecules along the β-sheet. We corroborated this unexpected scheme for β-strand organization using multiple two-dimensional NMR and 13C–13C dipolar recoupling experiments. Our findings indicate a previously unknown assembly pathway and inspire a suggestion as to why this aggregate does not grow to larger sizes.
中文翻译:

失配平行β-Sheets和反平行β-Sheets共存于淀粉样β(1-42)形成的150 kDa寡聚物中。
我们介绍了Alzheimer淀粉样β肽(Aβ(1-42))的42个残基变体的150 kDa寡聚体中的β链二级结构和链间组织的固态NMR测量。我们在先前的报告中建立了一个残基为30-42的β链,该残基排列成反平行的β-折叠。此处给出的新结果表明,有第二个β链由残基11-24形成。与预期相反,NMR数据表明,尽管在同一结构中同时存在一个反平行的β-折叠层,但第二条β-链仍组织成一个平行的β-折叠层。此外,通常观察到的淀粉样蛋白原纤维结构的配准平行β-折叠不适用于150 kDa低聚物中的残基11–24。而是 我们提供了三个残基的链间注册表移位的证据,这三个残基可能沿着β-折叠在相邻分子之间的方向交替。我们使用多个二维核磁共振和13 C– 13 C偶极耦合实验。我们的发现表明了以前未知的组装途径,并提出了关于为什么这种聚集体无法生长到更大尺寸的建议。











































 京公网安备 11010802027423号
京公网安备 11010802027423号