Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2020-05-26 , DOI: 10.1016/j.jfluchem.2020.109573 Ranjana Aggarwal , Suresh Kumar , Ashwani Mittal , Rachna Sadana , Vikas Dutt
|
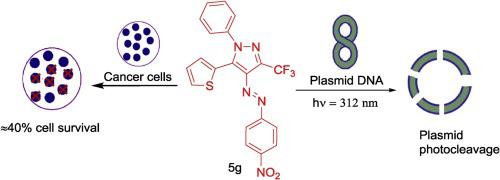
1-(2-Thienyl)-2-arylazo-4,4,4-trifluorobutane-1,3-diones (4), generated by the condensation of aryldiazonium salts (2) with 4,4,4-trifluoro-1-(2-thienyl)butane-1,3-dione (3), were used as common intermediates to synthesize regioisomeric 4-arylazo-1-phenyl-5(3)-(2-thienyl)-3(5)-trifluoromethylpyrazoles (5 and 6) and 4-arylazo-1-phenyl-3-(2-thienyl)-5-hydroxy-5-trifluoromethylpyrazolines (7) by reacting with phenylhydrazine in neutral conditions. All the synthesized compounds 4, 5, 6 and 7 were screened for their DNA photocleavage activity on pBR322 supercoiled DNA plasmid under UV radiation at λmax 312 nm without any additive. The results indicated that 3-trifluoromethyl isomers (5) photocleaves plasmid DNA better than the corresponding 5-trifluoromethylpyrazoles (6) and 5-hydroxy5-trifluoromethylpyrazolines (7). 4-(4-nitrophenylazo)-1-phenyl-5-(2-thienyl)-3-trifluoromethylpyrazole 5g, exhibited the best potential as DNA photocleaver, cleaving the supercoiled plasmid (Form-I) into smaller fragments at 20 μM of compound with 60 μM of DNA plasmid in DMSO. Selected seventeen compounds were further screened for their cytotoxicity against four cancer cell lines namely MCF-7, BT-474, SBALL and MOLT-4 and again compound 5g was identified as the most potent compound with an IC50 value of 8.6 ± 3.8 μM against MCF-7 cell line.
中文翻译:

4-芳基偶氮-1-苯基-3-(2-噻吩基)-5-羟基-5-三氟甲基吡唑啉和区域异构体4-芳基偶氮-1-苯基-5(3)-的合成,表征,体外DNA光裂解和细胞毒性研究(2-噻吩基)-3(5)-三氟甲基吡唑
1-(2-噻吩基)-2-芳基偶氮-4,4,4-三氟丁烷-1,3-二酮(4),是由芳基重氮盐(2)与4,4,4-三氟-1-缩合生成的(2-噻吩基)丁烷-1,3-二酮(3)用作常见的中间体,以合成区域异构的4-芳基偶氮-1-苯基-5(3)-(2-噻吩基)-3(5)-三氟甲基吡唑(5和6)和4-芳基偶氮-1-苯基-3-(2-噻吩基)-5-羟基-5-三氟甲基吡唑啉(7)通过在中性条件下与苯肼反应。所有合成的化合物4,5,6和7在没有任何添加剂的情况下,在λmax 312 nm的紫外线辐射下,筛选pBR322超螺旋DNA质粒的DNA光裂解活性。结果表明3-三氟甲基异构体(5)比相应的5-三氟甲基吡唑(6)和5-羟基5-三氟甲基吡唑啉(7)更好地裂解质粒DNA 。4-(4-硝基苯基偶氮)-1-苯基-5-(2-噻吩基)-3-三氟甲基吡唑5g,显示了作为DNA光切割机的最佳潜力,将超螺旋质粒(Form-I)在DMSO中与20μM的化合物和60μM的DNA质粒一起裂解成较小的片段。进一步筛选了所选的17种化合物对四种癌细胞系MCF-7,BT-474,SBALL和MOLT-4的细胞毒性,并再次确定化合物5g是最有效的化合物,其IC 50值为8.6±3.8μM。 MCF-7细胞系。











































 京公网安备 11010802027423号
京公网安备 11010802027423号