当前位置:
X-MOL 学术
›
Enzyme Microb. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biochemical and structural insights into an Ochrobactrum sp. CSL1 ribose-5-phosphate isomerase A and its roles in isomerization of rare sugars
Enzyme and Microbial Technology ( IF 3.4 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.enzmictec.2020.109604 Xin Ju 1 , Xinqi Xu 2 , Min Shen 1 , Xiaobing Mo 3 , Huan Fan 4 , Liangzhi Li 5
Enzyme and Microbial Technology ( IF 3.4 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.enzmictec.2020.109604 Xin Ju 1 , Xinqi Xu 2 , Min Shen 1 , Xiaobing Mo 3 , Huan Fan 4 , Liangzhi Li 5
Affiliation
|
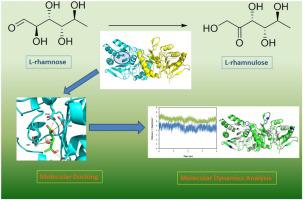
Rare sugars have received increasing attention due to their important applications as sweeteners and building blocks. The substrate specificity and catalytic properties of ribose-5-phosphate isomerase A (RpiA) in isomerization of rare sugars have not been extensively explored. In this study, an RpiA from Ochrobactrum sp. CSL1 was cloned and expressed in Escherichia coli. The biochemical and reaction features were explored and its broad substrate specificity was identified. A higher reaction rate in isomerizing l-rhamnose to l-rhamnulose by OsRpiA, compared with OsRpiB found in the same strain indicated higher efficiency in preparing rare sugars, which was verified by kinetics study. The 2.8 Å resolution structure of OsRpiA was then solved and used in subsequent molecular dynamics experiments, providing a possible explanation for its distinct substrate specificity. The present study highlighted the unique role of microbial RpiA in preparing rare sugars, and its structural information provided a reliable reference for further reaction mechanism research and enzyme engineering work.
中文翻译:

对 Ochrobactrum sp. 的生化和结构见解。CSL1 5-磷酸核糖异构酶A及其在稀有糖异构化中的作用
稀有糖类因其作为甜味剂和积木的重要应用而受到越来越多的关注。5-磷酸核糖异构酶 A (RpiA) 在稀有糖异构化中的底物特异性和催化特性尚未得到广泛研究。在这项研究中,来自 Ochrobactrum sp. 的 RpiA。CSL1 被克隆并在大肠杆菌中表达。探索了生化和反应特征,并确定了其广泛的底物特异性。与在同一菌株中发现的 OsRpiB 相比,OsRpiA 将 l-鼠李糖异构化为 l-鼠李酮糖的反应速率更高,表明制备稀有糖的效率更高,这通过动力学研究得到证实。OsRpiA 的 2.8 Å 分辨率结构随后被解析并用于后续的分子动力学实验,为其独特的底物特异性提供了可能的解释。本研究突出了微生物RpiA在制备稀有糖方面的独特作用,其结构信息为进一步的反应机理研究和酶工程工作提供了可靠的参考。
更新日期:2020-10-01
中文翻译:

对 Ochrobactrum sp. 的生化和结构见解。CSL1 5-磷酸核糖异构酶A及其在稀有糖异构化中的作用
稀有糖类因其作为甜味剂和积木的重要应用而受到越来越多的关注。5-磷酸核糖异构酶 A (RpiA) 在稀有糖异构化中的底物特异性和催化特性尚未得到广泛研究。在这项研究中,来自 Ochrobactrum sp. 的 RpiA。CSL1 被克隆并在大肠杆菌中表达。探索了生化和反应特征,并确定了其广泛的底物特异性。与在同一菌株中发现的 OsRpiB 相比,OsRpiA 将 l-鼠李糖异构化为 l-鼠李酮糖的反应速率更高,表明制备稀有糖的效率更高,这通过动力学研究得到证实。OsRpiA 的 2.8 Å 分辨率结构随后被解析并用于后续的分子动力学实验,为其独特的底物特异性提供了可能的解释。本研究突出了微生物RpiA在制备稀有糖方面的独特作用,其结构信息为进一步的反应机理研究和酶工程工作提供了可靠的参考。











































 京公网安备 11010802027423号
京公网安备 11010802027423号