当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synergistic effect between gold nanoparticles and Fe-doped γ-MnO2 toward enhanced aerobic selective oxidation of ethanol
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020-05-25 , DOI: 10.1039/d0cy00758g Panpan Wang 1, 2, 3, 4, 5 , Huimin Luo 1, 2, 3, 4, 5 , Jingwen Wang 4, 5, 6, 7 , Bo Han 4, 5, 6, 7 , Fuming Mei 1, 2, 3, 4, 5 , Peng Liu 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020-05-25 , DOI: 10.1039/d0cy00758g Panpan Wang 1, 2, 3, 4, 5 , Huimin Luo 1, 2, 3, 4, 5 , Jingwen Wang 4, 5, 6, 7 , Bo Han 4, 5, 6, 7 , Fuming Mei 1, 2, 3, 4, 5 , Peng Liu 1, 2, 3, 4, 5
Affiliation
|
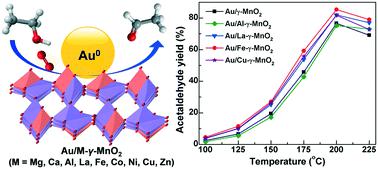
Developing synergistic catalysts between gold nanoparticles (AuNPs) and supports is crucial to enhance the catalytic efficiency of supported AuNP catalysts for the aerobic selective oxidation of ethanol to acetaldehyde (AC). In this study, for the first time, AuNPs supported on metal-doped γ-MnO2 (Au/M-γ-MnO2, M = Mg, Ca, Al, La, Fe, Co, Ni, Cu, Zn) catalysts with high gold dispersion were prepared by the colloidal deposition method and used in gas-phase ethanol oxidation to achieve up to 85% AC yield at 200 °C. It was found that the Au/M-γ-MnO2 catalysts show obviously higher reducibility and activity than the M-γ-MnO2 supports, and catalyst prereduction can further improve the catalytic activity. The strongest gold–support synergy was observed in the optimal Au/Fe-γ-MnO2 catalyst with a moderate Fe-doping amount, which can achieve the highest space–time-yield (∼3.2 g gcat−1 h−1) and outperform the previously reported supported AuNP catalysts at 200 °C. Kinetic isotope effect studies clearly suggest that the α-C–H cleavage is more rate-limiting than the O–H cleavage in ethanol oxidation and the surface adsorbed oxygen species on MnO2 is involved in the initial ethanol activation. By comparing the XPS spectra of the fresh and spent Au/Fe-γ-MnO2 catalysts, a novel Au–Mn–Fe synergy was proposed to account for its superior catalytic activity. The Mn2+ and Fe2+ defect-enriched catalyst interface is thought to facilitate the activation of O2 and ethanol at lower temperature.
中文翻译:

金纳米粒子与掺铁的γ-MnO2协同作用增强乙醇的好氧选择性氧化
在金纳米颗粒(AuNPs)和载体之间开发协同催化剂对于提高负载的AuNP催化剂将乙醇有氧选择性氧化为乙醛(AC)的催化效率至关重要。在这项研究中,在第一次,支撑在金属掺杂的γ-MnO的金纳米颗粒2(AU / M-γ-MnO的2,M =镁,钙,铝,镧,铁,钴,镍,铜,锌)催化剂通过胶体沉积法制备了具有高金分散度的金属,并将其用于气相乙醇氧化,以在200°C下获得高达85%的AC收率。结果发现,在Au / M-γ-的MnO 2种催化剂表现出比M-γ-MnO的显着高于还原性和活性2载体和催化剂的预还原可以进一步提高催化活性。在最佳观察到最强的金-载体的协同作用的Au / Fe基γ-MnO的2催化剂具有中等的Fe掺杂量,从而可以实现最高的空间-时间-产率(~3.2 GG猫-1 ħ -1)和在200°C下性能优于先前报道的负载型AuNP催化剂。动力学同位素效应研究清楚地表明,在乙醇氧化中,α-C–H裂解比O–H裂解更具限制性,并且MnO 2上的表面吸附氧物种参与了最初的乙醇活化。通过比较新鲜的XPS光谱和花费的Au / Fe基γ-MnO的2催化剂,提出了一种新型的Au-Mn-Fe协同作用,以说明其优越的催化活性。据认为,Mn 2+和Fe 2+富集缺陷的催化剂界面有助于在较低温度下活化O 2和乙醇。
更新日期:2020-07-06
中文翻译:

金纳米粒子与掺铁的γ-MnO2协同作用增强乙醇的好氧选择性氧化
在金纳米颗粒(AuNPs)和载体之间开发协同催化剂对于提高负载的AuNP催化剂将乙醇有氧选择性氧化为乙醛(AC)的催化效率至关重要。在这项研究中,在第一次,支撑在金属掺杂的γ-MnO的金纳米颗粒2(AU / M-γ-MnO的2,M =镁,钙,铝,镧,铁,钴,镍,铜,锌)催化剂通过胶体沉积法制备了具有高金分散度的金属,并将其用于气相乙醇氧化,以在200°C下获得高达85%的AC收率。结果发现,在Au / M-γ-的MnO 2种催化剂表现出比M-γ-MnO的显着高于还原性和活性2载体和催化剂的预还原可以进一步提高催化活性。在最佳观察到最强的金-载体的协同作用的Au / Fe基γ-MnO的2催化剂具有中等的Fe掺杂量,从而可以实现最高的空间-时间-产率(~3.2 GG猫-1 ħ -1)和在200°C下性能优于先前报道的负载型AuNP催化剂。动力学同位素效应研究清楚地表明,在乙醇氧化中,α-C–H裂解比O–H裂解更具限制性,并且MnO 2上的表面吸附氧物种参与了最初的乙醇活化。通过比较新鲜的XPS光谱和花费的Au / Fe基γ-MnO的2催化剂,提出了一种新型的Au-Mn-Fe协同作用,以说明其优越的催化活性。据认为,Mn 2+和Fe 2+富集缺陷的催化剂界面有助于在较低温度下活化O 2和乙醇。











































 京公网安备 11010802027423号
京公网安备 11010802027423号