当前位置:
X-MOL 学术
›
Anal. Methods
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A simple and sensitive LC-MS/MS method for determination and quantification of potential genotoxic impurities in the ceritinib active pharmaceutical ingredient.
Analytical Methods ( IF 2.7 ) Pub Date : 2020-05-25 , DOI: 10.1039/d0ay00511h Mia Antolčić 1 , Mislav Runje 1 , Nives Galić 2
Analytical Methods ( IF 2.7 ) Pub Date : 2020-05-25 , DOI: 10.1039/d0ay00511h Mia Antolčić 1 , Mislav Runje 1 , Nives Galić 2
Affiliation
|
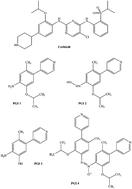
A liquid chromatography-tandem mass spectrometry (LC-MS/MS) method was used for quantification of four potential genotoxic impurities (PGIs) in the ceritinib active pharmaceutical ingredient. Chromatographic separation was achieved using a YMC-Triart C18 column, with 0.1% formic acid in water as mobile phase A and acetonitrile as mobile phase B in gradient elution mode at a 0.5 mL min−1 flow rate. Quantification of impurities was carried out using triple quadrupole mass detection with electrospray ionization in multiple reaction monitoring mode. The method was fully validated with good linearity over the concentration range of 0.5–5.0 ppm of the ceritinib test concentration for all four PGIs. The correlation coefficient obtained in each case was >0.998. The recoveries were found satisfactory over the range between 83.7 and 107.3% for all selected impurities. The developed method was able to quantitate all four PGIs at a concentration level of 1 ng mL−1 (0.5 ppm with respect to 2 mg mL−1 ceritinib).
中文翻译:

一种简单而灵敏的LC-MS / MS方法,用于确定和量化塞立替尼活性药物成分中潜在的遗传毒性杂质。
液相色谱-串联质谱(LC-MS / MS)方法用于定量定量测定ceritinib活性药物成分中的四种潜在遗传毒性杂质(PGI)。使用YMC-Triart C18色谱柱进行色谱分离,以0.1 mL甲酸的水溶液作为流动相A,乙腈作为流动相B在梯度洗脱模式下以0.5 mL min -1洗脱流量。杂质的定量使用多重反应监测模式下的三重四极杆质量检测和电喷雾电离进行。在所有四个PGI的赛立替尼测试浓度的0.5–5.0 ppm的浓度范围内,该方法均具有良好的线性,经过充分验证。在每种情况下获得的相关系数> 0.998。发现所有选定杂质的回收率在83.7%至107.3%的范围内令人满意。所开发的方法能够以1 ng mL -1的浓度(相对于2 mg mL -1 ceritinib为0.5 ppm)对所有四个PGI进行定量。
更新日期:2020-07-02
中文翻译:

一种简单而灵敏的LC-MS / MS方法,用于确定和量化塞立替尼活性药物成分中潜在的遗传毒性杂质。
液相色谱-串联质谱(LC-MS / MS)方法用于定量定量测定ceritinib活性药物成分中的四种潜在遗传毒性杂质(PGI)。使用YMC-Triart C18色谱柱进行色谱分离,以0.1 mL甲酸的水溶液作为流动相A,乙腈作为流动相B在梯度洗脱模式下以0.5 mL min -1洗脱流量。杂质的定量使用多重反应监测模式下的三重四极杆质量检测和电喷雾电离进行。在所有四个PGI的赛立替尼测试浓度的0.5–5.0 ppm的浓度范围内,该方法均具有良好的线性,经过充分验证。在每种情况下获得的相关系数> 0.998。发现所有选定杂质的回收率在83.7%至107.3%的范围内令人满意。所开发的方法能够以1 ng mL -1的浓度(相对于2 mg mL -1 ceritinib为0.5 ppm)对所有四个PGI进行定量。











































 京公网安备 11010802027423号
京公网安备 11010802027423号