当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical Selective Recovery of Heavy Metal Vanadium Oxyanion from Continuously Flowing Aqueous Streams.
ChemSusChem ( IF 7.5 ) Pub Date : 2020-05-25 , DOI: 10.1002/cssc.202001094 Ali Hemmatifar 1 , Nil Ozbek 1 , Cameron Halliday 1 , T Alan Hatton 1
ChemSusChem ( IF 7.5 ) Pub Date : 2020-05-25 , DOI: 10.1002/cssc.202001094 Ali Hemmatifar 1 , Nil Ozbek 1 , Cameron Halliday 1 , T Alan Hatton 1
Affiliation
|
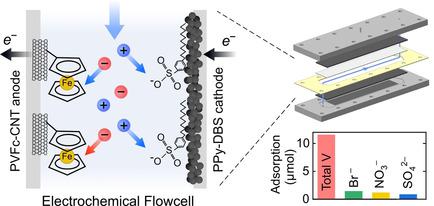
An electrochemical flow cell with redox‐active electrodes was used for selective removal and recovery of vanadium(V) oxyanions from aqueous streams. The cell relies on intrinsic affinity of the redox‐active polymer poly(vinyl)ferrocene (PVFc) and demonstrates selectivity of >10 towards vanadium compared to a background electrolyte in 40‐fold abundance. We demonstrate highly selective vanadium removal in the presence of various competing anions (i.e., fluoride, bromide, nitrate, and sulfate). Surface elemental analysis reveals significant correlation between PVFc moieties and vanadium‐rich regions after adsorption, corroborating the central role of PVFc modulation on vanadium separation. We further propose a vanadium speciation mechanism in which high and low pH environments during adsorption and desorption steps favor formation of, respectively, H2VO3−/ HVO42− and H2VO3−/ H3VO4/ VO2+. Results have implications for the development and optimization of flow devices, as per our observations, excessively low pH environments during desorption can lead to subsequent re‐adsorption of cationic vanadium(V).
中文翻译:

从连续流动的水流中电化学选择性回收重金属钒氧阴离子。
带有氧化还原活性电极的电化学流动池用于选择性去除和回收水流中的钒(V)氧阴离子。该电池依赖于氧化还原活性聚合物聚(乙烯基)二茂铁 (PVFc) 的内在亲和力,与丰度为 40 倍的背景电解质相比,对钒的选择性 >10。我们证明了在各种竞争性阴离子(即氟化物、溴化物、硝酸盐和硫酸盐)存在下的高选择性除钒。表面元素分析揭示了PVFc部分与吸附后富钒区域之间的显着相关性,证实了PVFc调制在钒分离中的核心作用。我们进一步提出了钒形态形成机制,其中吸附和解吸步骤期间的高和低pH环境分别有利于形成H 2 VO 3 − / HVO 4 2−和H 2 VO 3 − / H 3 VO 4 / VO 2 +。结果对流动装置的开发和优化具有影响,根据我们的观察,解吸过程中过低的 pH 环境可能导致随后阳离子钒 (V) 的重新吸附。
更新日期:2020-05-25
中文翻译:

从连续流动的水流中电化学选择性回收重金属钒氧阴离子。
带有氧化还原活性电极的电化学流动池用于选择性去除和回收水流中的钒(V)氧阴离子。该电池依赖于氧化还原活性聚合物聚(乙烯基)二茂铁 (PVFc) 的内在亲和力,与丰度为 40 倍的背景电解质相比,对钒的选择性 >10。我们证明了在各种竞争性阴离子(即氟化物、溴化物、硝酸盐和硫酸盐)存在下的高选择性除钒。表面元素分析揭示了PVFc部分与吸附后富钒区域之间的显着相关性,证实了PVFc调制在钒分离中的核心作用。我们进一步提出了钒形态形成机制,其中吸附和解吸步骤期间的高和低pH环境分别有利于形成H 2 VO 3 − / HVO 4 2−和H 2 VO 3 − / H 3 VO 4 / VO 2 +。结果对流动装置的开发和优化具有影响,根据我们的观察,解吸过程中过低的 pH 环境可能导致随后阳离子钒 (V) 的重新吸附。











































 京公网安备 11010802027423号
京公网安备 11010802027423号