当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Manganese-Catalyzed anti-Selective Asymmetric Hydrogenation of α-Substituted β-Ketoamides.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-05-25 , DOI: 10.1002/anie.202006383 Linli Zhang 1 , Zheng Wang 1 , Zhaobin Han 1 , Kuiling Ding 1, 2, 3
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-05-25 , DOI: 10.1002/anie.202006383 Linli Zhang 1 , Zheng Wang 1 , Zhaobin Han 1 , Kuiling Ding 1, 2, 3
Affiliation
|
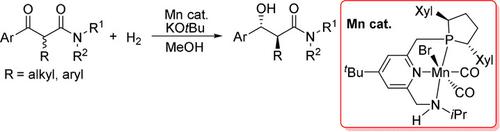
A Mn‐catalyzed diastereo‐ and enantioselective hydrogenation of α‐substituted β‐ketoamides has been realized for the first time under dynamic kinetic resolution conditions. anti‐α‐Substituted β‐hydroxy amides, which are useful building blocks for the synthesis of bioactive molecules and chiral drugs, were prepared in high yields with excellent selectivity (up to >99 % dr and >99 % ee) and unprecedentedly high activity (TON up to 10000). The origin of the excellent stereoselectivity was clarified by DFT calculations.
中文翻译:

锰催化的α-取代的β-酮酰胺的抗选择性不对称加氢反应。
在动态动力学拆分条件下,首次实现了Mn催化的α-取代的β-酮酰胺的非对映和对映选择性氢化。抗-α-取代的β-羟基酰胺是生物活性分子和手性药物合成的有用组成部分,以高收率,高选择性(高达> 99%dr和> 99%ee)和前所未有的高活性制备。(TON最高10000)。出色的立体选择性的起源已通过DFT计算得以阐明。
更新日期:2020-05-25
中文翻译:

锰催化的α-取代的β-酮酰胺的抗选择性不对称加氢反应。
在动态动力学拆分条件下,首次实现了Mn催化的α-取代的β-酮酰胺的非对映和对映选择性氢化。抗-α-取代的β-羟基酰胺是生物活性分子和手性药物合成的有用组成部分,以高收率,高选择性(高达> 99%dr和> 99%ee)和前所未有的高活性制备。(TON最高10000)。出色的立体选择性的起源已通过DFT计算得以阐明。











































 京公网安备 11010802027423号
京公网安备 11010802027423号