当前位置:
X-MOL 学术
›
J. Mol. Struct.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel monoanionic diphenate-nicotinamide/N,N-diethylnicotinamide complexes of NiII, ZnII. Synthesis, structural investigations and hydrogen adsorption study
Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.molstruc.2020.128514 Ömer Yurdakul , Zarife Sibel Şahin , Dursun Ali Köse , Onur Şahin , Fatih Akkurt
Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.molstruc.2020.128514 Ömer Yurdakul , Zarife Sibel Şahin , Dursun Ali Köse , Onur Şahin , Fatih Akkurt
|
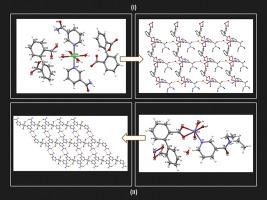
Abstract Mixed ligand complexes containing monoanionic diphenate (Hdiphen-), dianionic diphenate (diphen2−)-nicotinamide (nic)/N,N-diethylnicotinamide (denic) ligands of Ni(II) and Zn(II) transition metal cations were synthesized, their structural characterizations and molecular simulation for hydrogen adsorption were performed. It is the salt type coordination compound of the Ni(II) complex, complementing the metal octahedral environment with 2 mol of nic and 4 mol of aqua ligands. Two moles of Hdiphen- ligand is monoanionic located outside the coordination sphere and provides the charge equivalence of the complex. In the Zn(II) complex structure, denic and diphen2− ligand is located within the coordination sphere. In the Zn(II) coordination compound structure, diphen2− ligands, which are located in the structure of a bridge between two different metal centres, make the structure polymeric. The binding properties of complexes to metal cations are also elucidated by infrared spectra. It has also been clarified whether the diphenate ligand is bonded to monovalent or bivalent. The mixed ligand complexes of diphenate [Ni(nic)(H2O)4].2(Hdiphen) (I) and [Zn(denic)(diphen)(H2O)]·H2O (II) distorted octahedral, and they have P-1 space group and triclinic crystal systems. The thermal decomposition of the complexes begins with dehydration steps and continues with the decomposition of organic ligands. As a result of the thermal analysis, it is determined that the oxides of the relevant metal cations remain as the final decomposition products of the molecules. The hydrogen storage of nickel complex is 0.153 wt% and the zinc complex is 0.289 wt% at 77 K and 10 bars.
中文翻译:

NiII、ZnII 的新型单阴离子二酚盐-烟酰胺/N,N-二乙基烟酰胺配合物。合成、结构研究和氢吸附研究
摘要 合成了含有 Ni(II) 和 Zn(II) 过渡金属阳离子的单阴离子联苯酸盐 (Hdiphen-)、双阴离子联苯酸盐 (diphen2-)-烟酰胺 (nic)/N,N-二乙基烟酰胺 (denic) 配体的混合配体配合物,进行了氢吸附的结构表征和分子模拟。它是 Ni(II) 配合物的盐型配位化合物,用 2 mol nic 和 4 mol 水配体补充金属八面体环境。两摩尔的 Hdiphen-配体是位于配位球外的单阴离子,并提供复合物的电荷等价性。在 Zn(II) 复合结构中,denic 和 diphen2− 配体位于配位球内。在 Zn(II) 配位化合物结构中,diphen2− 配体,位于两个不同金属中心之间的桥梁结构中,使结构聚合。配合物与金属阳离子的结合特性也可以通过红外光谱来阐明。还阐明了二苯甲酸酯配体是与一价还是二价键合。苯甲酸酯[Ni(nic)(H2O)4].2(Hdiphen)(I)和[Zn(denic)(diphen)(H2O)]·H2O(II)的混合配体配合物扭曲八面体,它们有P- 1 空间群和三斜晶系。配合物的热分解从脱水步骤开始,并随着有机配体的分解而继续。作为热分析的结果,确定相关金属阳离子的氧化物作为分子的最终分解产物保留。镍配合物的储氢量为0。
更新日期:2020-10-01
中文翻译:

NiII、ZnII 的新型单阴离子二酚盐-烟酰胺/N,N-二乙基烟酰胺配合物。合成、结构研究和氢吸附研究
摘要 合成了含有 Ni(II) 和 Zn(II) 过渡金属阳离子的单阴离子联苯酸盐 (Hdiphen-)、双阴离子联苯酸盐 (diphen2-)-烟酰胺 (nic)/N,N-二乙基烟酰胺 (denic) 配体的混合配体配合物,进行了氢吸附的结构表征和分子模拟。它是 Ni(II) 配合物的盐型配位化合物,用 2 mol nic 和 4 mol 水配体补充金属八面体环境。两摩尔的 Hdiphen-配体是位于配位球外的单阴离子,并提供复合物的电荷等价性。在 Zn(II) 复合结构中,denic 和 diphen2− 配体位于配位球内。在 Zn(II) 配位化合物结构中,diphen2− 配体,位于两个不同金属中心之间的桥梁结构中,使结构聚合。配合物与金属阳离子的结合特性也可以通过红外光谱来阐明。还阐明了二苯甲酸酯配体是与一价还是二价键合。苯甲酸酯[Ni(nic)(H2O)4].2(Hdiphen)(I)和[Zn(denic)(diphen)(H2O)]·H2O(II)的混合配体配合物扭曲八面体,它们有P- 1 空间群和三斜晶系。配合物的热分解从脱水步骤开始,并随着有机配体的分解而继续。作为热分析的结果,确定相关金属阳离子的氧化物作为分子的最终分解产物保留。镍配合物的储氢量为0。











































 京公网安备 11010802027423号
京公网安备 11010802027423号