JACC: Heart Failure ( IF 10.3 ) Pub Date : 2020-05-25 , DOI: 10.1016/j.jchf.2020.02.010 João Pedro Ferreira 1 , Pardeep S Jhund 2 , Kévin Duarte 3 , Brian L Claggett 4 , Scott D Solomon 4 , Stuart Pocock 5 , Mark C Petrie 2 , Faiez Zannad 6 , John J V McMurray 2
|
Objectives
The purpose of this study was to compare the win ratio (WR) with the corresponding hazard ratios (HRs) and 1/HR.
Background
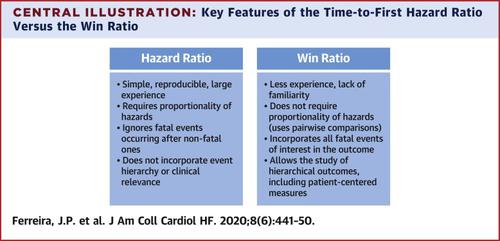
The primary outcome in many cardiovascular trials is a composite that includes nonfatal and fatal events. The time-to-first event analysis gives equal statistical weighting to each component event. The WR, which takes into account the clinical importance and timing of the outcomes, has been suggested as an alternative approach.
Methods
Cox proportional hazards models and WR.
Results
In the these trials (n = 16) the WR and HR differed only slightly. For example, in the PARADIGM-HF (sacubitril/valsartan vs. enalapril), the primary outcome of time to first heart failure hospitalization (HFH) or cardiovascular death (CVD) and use of the Cox model gave a 1/HR of 1.25 (95% confidence interval [CI]: 1.12 to 1. 41; z-score = 4.8). Using WR for testing this composite in the hierarchical order of CVD and HFH gave a WR of 1.27 (95% CI: 1.15 to 1.39; z-score = 4.7), reflecting an effect similar to that of sacubitril/valsartan therapy on CVD and HFH. In the DIG (digoxin vs. placebo) trial, the outcome of time-to-first HFH or CVD using Cox gave a 1/HR of 1.18 (95% CI: 1.10 to 1.27; z-score = 4.5). Using the WR for testing this composite in the hierarchical order of CVD and HFH gave a WR of 1.14 (95% CI: 1.05 to 1.20; z-score = 3.1), reflecting a larger effect of digoxin on HFH than on CVD. Several other trials and endpoints including patient-reported measurements were studied.
Conclusions
In 16 large cardiovascular outcome trials, HR and WR provided similar estimates of treatment effects. The WR allows prioritization of fatal outcomes and the hierarchical testing of broader composite endpoints including patient-reported outcomes. In this way, the WR allows for the incorporation of patient-centered and other outcomes, while prioritizing the competing risk of death and hospital admission.
中文翻译:

在心血管试验中使用胜率。
目标
这项研究的目的是比较获胜率(WR)与相应的危险比(HRs)和1 / HR。
背景
许多心血管试验的主要结果是包括非致命和致命事件的综合结果。首次事件时间分析为每个组件事件提供了相等的统计权重。已经提出了WR,它考虑了临床重要性和结果的时机,是一种替代方法。
方法
考克斯比例风险模型和WR。
结果
在这些试验(n = 16)中,WR和HR仅略有不同。例如,在PARADIGM-HF(沙比特比/缬沙坦与依那普利)中,首次心衰住院(HFH)或心血管死亡(CVD)的时间的主要结果以及使用Cox模型得出的1 / HR为1.25( 95%置信区间[CI]:1.12至1. 41; z得分= 4.8)。使用WR按CVD和HFH的分层顺序测试该复合材料的WR为1.27(95%CI:1.15至1.39; z分数= 4.7),反映出与沙比特利/缬沙坦疗法对CVD和HFH相似的作用。在DIG(地高辛与安慰剂)试验中,使用Cox进行首次HFH或CVD的时间得出的1 / HR为1.18(95%CI:1.10至1.27; z评分= 4.5)。使用WR按照CVD和HFH的分层顺序测试此复合材料,得出的WR为1.14(95%CI:1.05至1.20; z分数= 3.1),反映了地高辛对HFH的作用大于对CVD的作用。研究了其他几个试验和终点,包括患者报告的测量结果。
结论
在16个大型心血管结局试验中,HR和WR提供了相似的治疗效果评估。WR允许对致命结果进行优先级排序,并对包括患者报告的结果在内的更广泛的复合终点进行分层测试。这样,WR可以纳入以患者为中心的结果和其他结果,同时优先考虑死亡和住院的竞争风险。











































 京公网安备 11010802027423号
京公网安备 11010802027423号