Journal of Structural Biology ( IF 3.0 ) Pub Date : 2020-05-23 , DOI: 10.1016/j.jsb.2020.107534 Juergen Linder 1 , Enrico Hupfeld 1 , Michael Weyand 1 , Clemens Steegborn 1 , Sébastien Moniot 1
|
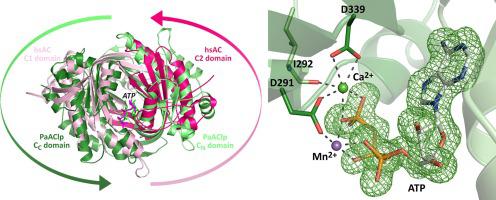
In many organisms, the ubiquitous second messenger cAMP is formed by at least one member of the adenylyl cyclase (AC) Class III. These ACs feature a conserved dimeric catalytic core architecture, either through homodimerization or through pseudo-heterodimerization of a tandem of two homologous catalytic domains, C1 and C2, on a single protein chain. The symmetric core features two active sites, but in the C1-C2 tandem one site degenerated into a regulatory center. Analyzing bacterial AC sequences, we identified a Pseudomonas aeruginosa AC-like protein (PaAClp) that shows a surprising swap of the catalytic domains, resulting in an unusual C2-C1 arrangement. We cloned and recombinantly produced PaAClp. The protein bound nucleotides but showed no AC or guanylyl cyclase activity, even in presence of a variety of stimulating ligands of other ACs. Solving the crystal structure of PaAClp revealed an overall structure resembling active class III ACs but pronounced shifts of essential catalytic residues and structural elements. The structure contains a tightly bound ATP, but in a binding mode not suitable for cAMP formation or ATP hydrolysis, suggesting that PaAClp acts as an ATP-binding protein.
中文翻译:

来自铜绿假单胞菌的 III 类腺苷酸环化酶样 ATP 结合蛋白的晶体结构。
在许多生物体中,普遍存在的第二信使 cAMP 是由至少一个腺苷酸环化酶 (AC) III 类成员形成的。这些 AC 具有保守的二聚催化核心结构,通过同源二聚化或通过单个蛋白质链上两个同源催化结构域 C1 和 C2 串联的假异二聚化。对称核心具有两个活性位点,但在 C1-C2 串联中,一个位点退化为调节中心。分析细菌 AC 序列,我们确定了铜绿假单胞菌AC 样蛋白 (PaAClp) 显示出令人惊讶的催化结构域交换,导致不寻常的 C2-C1 排列。我们克隆并重组生产了 PaAClp。蛋白质结合核苷酸,但没有表现出 AC 或鸟苷酸环化酶活性,即使在其他 AC 的各种刺激配体存在的情况下。解决 PaAClp 的晶体结构揭示了类似于活性 III 类 AC 的整体结构,但基本催化残基和结构元素发生了显着变化。该结构包含紧密结合的 ATP,但处于不适合 cAMP 形成或 ATP 水解的结合模式,表明 PaAClp 充当 ATP 结合蛋白。











































 京公网安备 11010802027423号
京公网安备 11010802027423号