Chemico-Biological Interactions ( IF 4.7 ) Pub Date : 2020-05-23 , DOI: 10.1016/j.cbi.2020.109139 Martijn C de Koning 1 , Gabriele Horn 2 , Franz Worek 2 , Marco van Grol 1
|
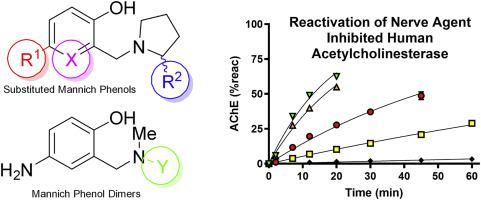
Since several decades oximes have been used as part of treatment of nerve agent intoxication with the aim to restore the biological function of the enzyme acetylcholinesterase after its covalent inhibition by organophosphorus compounds such as pesticides and nerve agents. Recent findings have illustrated that, besides oximes, certain Mannich phenols can reactivate the inhibited enzyme very effectively, and may therefore represent an attractive complementary class of reactivators. In this paper we further probe the effect of structural variation on the in vitro efficacy of Mannich phenol based reactivators. Thus, we present the synthesis of 14 compounds that are close variants of the previously reported 4-amino-2-(1-pyrrolidinylmethyl)-phenol, a very effective non-oxime reactivator, and 3 dimeric Mannich phenols. All compounds were assessed for their ability to reactivate human acetylcholinesterase inhibited by the nerve agents VX, tabun, sarin, cyclosarin and paraoxon in vitro. It was confirmed that the potency of the compounds is highly sensitive to small structural changes, leading to diminished reactivation potency in many cases. However, the presence of 4-substituted alkylamine substituents (as exemplified with the 4-benzylamine-variant) was tolerated. More surprisingly, the dimeric compounds demonstrated non-typical behavior and displayed some reactivation potency as well. Both findings may open up new avenues for designing more effective non-oxime reactivators.
中文翻译:

新型非肟的合成和体外评估,用于神经药抑制人乙酰胆碱酯酶的活化。
几十年来,肟一直被用作治疗神经毒症中毒的一部分,目的是在被有机磷化合物(如农药和神经毒剂)共价抑制后恢复乙酰胆碱酯酶的生物学功能。最近的发现表明,除肟外,某些曼尼希酚还可以非常有效地重新活化被抑制的酶,因此可能代表了有吸引力的互补类活化剂。在本文中,我们进一步探讨了结构变异对曼尼希酚基活化剂体外功效的影响。因此,我们提出了14种化合物的合成,这些化合物是先前报道的4-氨基-2-(1-吡咯烷基甲基)苯酚,非常有效的非肟活化剂和3种二聚曼尼希酚的近似变体。评估了所有化合物在体外重新激活神经药VX,塔邦,沙林,沙林,环沙蛋白和对氧磷抑制的人乙酰胆碱酯酶的能力。证实了化合物的效力对小的结构变化高度敏感,从而在许多情况下导致再活化效力降低。然而,容许存在4-取代的烷基胺取代基(以4-苄基胺变体为例)。更令人惊讶的是,二聚体化合物表现出非典型的行为并且还显示出一些再活化潜能。两项发现都可能为设计更有效的非肟类活化剂开辟新途径。证实了化合物的效力对小的结构变化高度敏感,从而在许多情况下导致再活化效力降低。然而,存在4-取代的烷基胺取代基(以4-苄基胺变体为例)的存在。更令人惊讶的是,二聚体化合物表现出非典型的行为并且还显示出一些再活化潜能。两项发现都可能为设计更有效的非肟类活化剂开辟新途径。证实了化合物的效力对小的结构变化高度敏感,从而在许多情况下导致再活化效力降低。然而,存在4-取代的烷基胺取代基(以4-苄基胺变体为例)的存在。更令人惊讶的是,二聚体化合物表现出非典型的行为并且还显示出一些再活化潜能。两项发现都可能为设计更有效的非肟类活化剂开辟新途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号