当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solvent-Free N-Alkylation and Dehydrogenative Coupling Catalyzed by a Highly Active Pincer-Nickel Complex
Organometallics ( IF 2.5 ) Pub Date : 2020-05-22 , DOI: 10.1021/acs.organomet.0c00233 Vinay Arora 1 , Moumita Dutta 1 , Kanu Das 1 , Babulal Das 1 , Hemant Kumar Srivastava 1, 2 , Akshai Kumar 1, 3
Organometallics ( IF 2.5 ) Pub Date : 2020-05-22 , DOI: 10.1021/acs.organomet.0c00233 Vinay Arora 1 , Moumita Dutta 1 , Kanu Das 1 , Babulal Das 1 , Hemant Kumar Srivastava 1, 2 , Akshai Kumar 1, 3
Affiliation
|
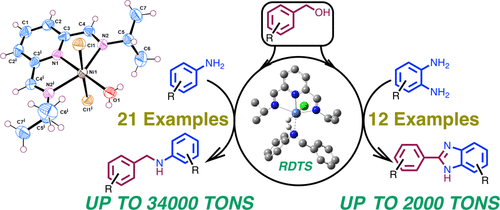
The synthesis and characterization of a pincer-nickel complex of the type (iPr2NNN)NiCl2(CH3CN) is reported here. We have demonstrated the utility of this pincer-nickel complex (0.02 and 0.002 mol %) for the catalytic N-alkylation of amines using various alcohols. Under solvent-free conditions, while the highest yield (ca. 90%) was obtained for the alkylation of 2-aminopyridine with naphthyl-1-methanol, excellent turnovers (34000 TONs) were observed for the alkylation of 2-aminopyridine with 4-methoxybenzyl alcohol. To demonstrate the synthetic utility of these systems, high-yield reactions (up to 98%) have been probed for representative substrates with a higher loading of the pincer-nickel catalyst (4 mol %). DFT studies indicate that while β-hydride elimination is the RDS for alcohol dehydrogenation, the N-alkylated product can be formed either via hydrogenation with a rate-determining σ-bond metathesis or by alcoholysis that has imine insertion as the RDS. All of the corresponding resting states have been observed by HRMS (ESI) analysis. The labeling experiments are also complementary to DFT studies and show evidence for the involvement of the benzylic C–H bond in the RDS with a kCHH/kCHD value of about 2.5. This method has been applied to accomplish efficient (2000 TONs) dehydrogenative coupling leading to various benzimidazoles.
中文翻译:

高活性钳形镍配合物催化的无溶剂N-烷基化和脱氢偶联
(i Pr2 NNN)NiCl 2(CH 3型)的钳镍配合物的合成与表征CN)在这里报告。我们已经证明了这种夹钳-镍配合物(0.02和0.002mol%)对于使用各种醇的胺的催化N-烷基化的实用性。在无溶剂条件下,用2-甲基萘-1-甲醇烷基化2-氨基吡啶的收率最高(约90%),而用4-甲基烷基磺酸2-氨基吡啶的烷基化收率高(34000 TONs)。甲氧基苄醇。为了证明这些系统的合成效用,已探查了具有较高负载的钳制镍催化剂(4摩尔%)的代表性底物的高产率反应(高达98%)。DFT研究表明,尽管消除β-氢化物是酒精脱氢的RDS,N-烷基化产物既可以通过加氢速率决定σ键的复分解反应进行氢化,也可以通过具有亚胺插入作为RDS的醇解反应形成。通过HRMS(ESI)分析已观察到所有相应的静止状态。标记实验还与DFT研究相辅相成,并显示了RDS中的苄基C–H键与DFT研究有关的证据。k CHH / k CHD值约为2.5。该方法已用于完成有效的(2000吨)脱氢偶联反应,从而制得各种苯并咪唑。
更新日期:2020-05-22
中文翻译:

高活性钳形镍配合物催化的无溶剂N-烷基化和脱氢偶联
(i Pr2 NNN)NiCl 2(CH 3型)的钳镍配合物的合成与表征CN)在这里报告。我们已经证明了这种夹钳-镍配合物(0.02和0.002mol%)对于使用各种醇的胺的催化N-烷基化的实用性。在无溶剂条件下,用2-甲基萘-1-甲醇烷基化2-氨基吡啶的收率最高(约90%),而用4-甲基烷基磺酸2-氨基吡啶的烷基化收率高(34000 TONs)。甲氧基苄醇。为了证明这些系统的合成效用,已探查了具有较高负载的钳制镍催化剂(4摩尔%)的代表性底物的高产率反应(高达98%)。DFT研究表明,尽管消除β-氢化物是酒精脱氢的RDS,N-烷基化产物既可以通过加氢速率决定σ键的复分解反应进行氢化,也可以通过具有亚胺插入作为RDS的醇解反应形成。通过HRMS(ESI)分析已观察到所有相应的静止状态。标记实验还与DFT研究相辅相成,并显示了RDS中的苄基C–H键与DFT研究有关的证据。k CHH / k CHD值约为2.5。该方法已用于完成有效的(2000吨)脱氢偶联反应,从而制得各种苯并咪唑。











































 京公网安备 11010802027423号
京公网安备 11010802027423号