当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Characterization of Polysorbate Ester Fractions and Implications in Protein Drug Product Stability.
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-05-22 , DOI: 10.1021/acs.molpharmaceut.0c00093 Anthony Tomlinson 1 , Isidro E Zarraga 1 , Barthélemy Demeule 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-05-22 , DOI: 10.1021/acs.molpharmaceut.0c00093 Anthony Tomlinson 1 , Isidro E Zarraga 1 , Barthélemy Demeule 1
Affiliation
|
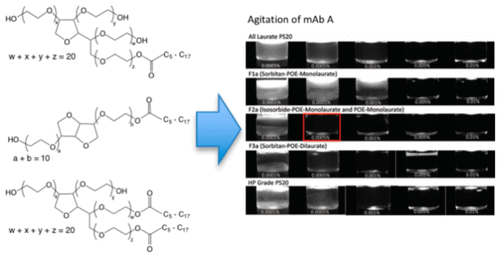
Polysorbates (PS) are commonly used surfactants in biopharmaceutical protein formulations. However, they are susceptible to a variety of degradation pathways, including chemical hydrolysis, oxidation, and enzymatic hydrolysis. Polysorbates are also heterogeneous mixtures, and it has been observed that the patterns of degradation can be strikingly different between the different pathways. Polysorbates (PS20 and PS80) were fractionated, and the fractions were characterized for their physicochemical properties, such as surface tension, micelle size, critical micelle concentration (CMC), and agitation protection for a monoclonal antibody (mAb). This report seeks to use this information to inform how these properties might change in polysorbates as they degrade in biopharmaceutical formulations. The physicochemical properties examined shed light on some of the differences between PS types and the different chemical components of polysorbates. Differences in physicochemical properties for fractionated polysorbates could help inform biopharmaceutical formulations that use PS surfactants. Importantly, they show that subspecies of PS20 are far more distinct from each other than those of PS80. Fractions of PS20 showed highly different critical micelle concentrations and effects on equilibrium surface tension. These differences, and possibly other untested parameters, led to vastly different protective effects for a model mAb under agitation stress. Additionally, the propensity of various PS fractions to form micelles can impact both polysorbate quantitation measurements, some of which rely on micellization, and the effective solubility of hydrophobic compounds (e.g., fatty acids) in the surfactant solution.
中文翻译:

聚山梨酯酯级分的表征及其对蛋白质药物产品稳定性的影响。
聚山梨酯(PS)是生物制药蛋白质制剂中常用的表面活性剂。但是,它们易受多种降解途径的影响,包括化学水解,氧化和酶促水解。聚山梨酯也是异质混合物,并且已经观察到,降解的模式在不同的途径之间可能截然不同。分离聚山梨酯(PS20和PS80),并对其馏分的理化性质进行表征,例如表面张力,胶束大小,临界胶束浓度(CMC)和单克隆抗体(mAb)的搅拌保护。本报告试图利用这些信息来告知聚山梨酯在生物制药配方中降解时这些特性可能会如何变化。检查的理化性质揭示了PS类型与聚山梨酯的不同化学成分之间的某些差异。分馏的聚山梨酸酯的理化性质差异可能有助于告知使用PS表面活性剂的生物药物制剂。重要的是,它们表明PS20的亚种比PS80的亚种相差很多。PS20的馏分显示出非常不同的临界胶束浓度和对平衡表面张力的影响。这些差异以及可能的其他未经测试的参数,导致模型mAb在搅拌应力下的保护作用大不相同。此外,各种PS馏分形成胶束的倾向可能会影响两种聚山梨酯的定量测量,其中一些依赖胶束化,
更新日期:2020-07-06
中文翻译:

聚山梨酯酯级分的表征及其对蛋白质药物产品稳定性的影响。
聚山梨酯(PS)是生物制药蛋白质制剂中常用的表面活性剂。但是,它们易受多种降解途径的影响,包括化学水解,氧化和酶促水解。聚山梨酯也是异质混合物,并且已经观察到,降解的模式在不同的途径之间可能截然不同。分离聚山梨酯(PS20和PS80),并对其馏分的理化性质进行表征,例如表面张力,胶束大小,临界胶束浓度(CMC)和单克隆抗体(mAb)的搅拌保护。本报告试图利用这些信息来告知聚山梨酯在生物制药配方中降解时这些特性可能会如何变化。检查的理化性质揭示了PS类型与聚山梨酯的不同化学成分之间的某些差异。分馏的聚山梨酸酯的理化性质差异可能有助于告知使用PS表面活性剂的生物药物制剂。重要的是,它们表明PS20的亚种比PS80的亚种相差很多。PS20的馏分显示出非常不同的临界胶束浓度和对平衡表面张力的影响。这些差异以及可能的其他未经测试的参数,导致模型mAb在搅拌应力下的保护作用大不相同。此外,各种PS馏分形成胶束的倾向可能会影响两种聚山梨酯的定量测量,其中一些依赖胶束化,











































 京公网安备 11010802027423号
京公网安备 11010802027423号