当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reactivity of p-Coumaroyl Groups in Lignin upon Laccase and Laccase/HBT Treatments
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-05-22 , DOI: 10.1021/acssuschemeng.0c02376 Roelant Hilgers 1 , Mirjam A. Kabel 1 , Jean-Paul Vincken 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-05-22 , DOI: 10.1021/acssuschemeng.0c02376 Roelant Hilgers 1 , Mirjam A. Kabel 1 , Jean-Paul Vincken 1
Affiliation
|
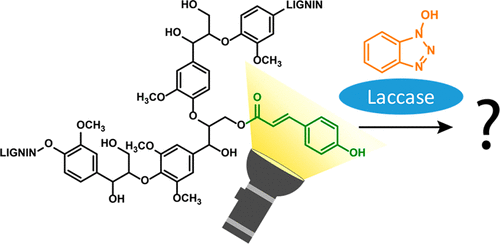
Laccase–mediator systems (LMS) are potential green tools for oxidative degradation and modification of lignin. Although LMS convert both phenolic and nonphenolic lignin structures, phenolic structures are more prone to react. Remarkably, in a previous study on laccase/HBT treatment of grasses, we observed the accumulation of p-coumaroyl moieties in residual lignin, even though such groups are free phenolic structures. To provide more insights into this apparent paradox, here, we studied the reactivity of p-coumaroyl groups in lignin and model compounds using HSQC NMR and RP-UHPLC-PDA-MSn, respectively. It was found that a p-coumaroylated model compound (VBG-pCA), in contrast to its nonacylated analogue, was rapidly converted by laccase and laccase/HBT, resulting in oxidative coupling and HBT-mediated degradation, respectively. The high reactivity of VBG-pCA was related to the phenolic character of the p-coumaroyl group. Upon laccase/HBT treatment of two grass lignin isolates, p-coumaroyl groups accumulated in residual lignin, indicating that p-coumaroyl groups in polymeric lignin display different reactivity than those in model compounds. On the basis of additional experiments, we propose that p-coumaroyl groups in lignin polymers can be oxidized by laccase/HBT but undergo HSQC-undetectable radical coupling or redox reactions rather than degradation.
中文翻译:

漆酶和漆酶/ HBT处理后木质素中对-香豆酰基的反应性
漆酶介体系统(LMS)是潜在的绿色工具,可用于木质素的氧化降解和修饰。尽管LMS可以同时转换酚醛和非酚醛木质素结构,但酚醛结构更容易发生反应。值得注意的是,在先前对漆酶/ HBT处理草的研究中,我们观察到残留的木质素中对-香豆酰基部分的积累,即使这些基团是游离酚结构。为了提供对此明显悖论的更多见解,在这里,我们分别使用HSQC NMR和RP-UHPLC-PDA-MS n研究了木质素和模型化合物中对香豆酰基的反应性。发现对-香豆素化的模型化合物(VBG- p与未酰化的类似物相反,CA)被漆酶和漆酶/ HBT迅速转化,分别导致氧化偶联和HBT介导的降解。VBG- p CA的高反应性与对-香豆酰基的酚特性有关。对两种草木素分离物进行漆酶/ HBT处理后,对-香豆酰基在残留的木质素中积累,表明聚合木素中的对香豆酰基显示出与模型化合物不同的反应性。在其他实验的基础上,我们提出木质素聚合物中的对香豆酰基可以被漆酶/ HBT氧化,但会经历HSQC不可检测的自由基偶联或氧化还原反应,而不是降解。
更新日期:2020-05-22
中文翻译:

漆酶和漆酶/ HBT处理后木质素中对-香豆酰基的反应性
漆酶介体系统(LMS)是潜在的绿色工具,可用于木质素的氧化降解和修饰。尽管LMS可以同时转换酚醛和非酚醛木质素结构,但酚醛结构更容易发生反应。值得注意的是,在先前对漆酶/ HBT处理草的研究中,我们观察到残留的木质素中对-香豆酰基部分的积累,即使这些基团是游离酚结构。为了提供对此明显悖论的更多见解,在这里,我们分别使用HSQC NMR和RP-UHPLC-PDA-MS n研究了木质素和模型化合物中对香豆酰基的反应性。发现对-香豆素化的模型化合物(VBG- p与未酰化的类似物相反,CA)被漆酶和漆酶/ HBT迅速转化,分别导致氧化偶联和HBT介导的降解。VBG- p CA的高反应性与对-香豆酰基的酚特性有关。对两种草木素分离物进行漆酶/ HBT处理后,对-香豆酰基在残留的木质素中积累,表明聚合木素中的对香豆酰基显示出与模型化合物不同的反应性。在其他实验的基础上,我们提出木质素聚合物中的对香豆酰基可以被漆酶/ HBT氧化,但会经历HSQC不可检测的自由基偶联或氧化还原反应,而不是降解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号