当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electroreduction of Nitrates, Nitrites, and Gaseous Nitrogen Oxides: A Potential Source of Ammonia in Dinitrogen Reduction Studies
ACS Energy Letters ( IF 19.3 ) Pub Date : 2020-05-22 , DOI: 10.1021/acsenergylett.0c00924 Jaecheol Choi , Hoang-Long Du , Cuong Ky Nguyen , Bryan H. R. Suryanto , Alexandr N. Simonov , Douglas R. MacFarlane
ACS Energy Letters ( IF 19.3 ) Pub Date : 2020-05-22 , DOI: 10.1021/acsenergylett.0c00924 Jaecheol Choi , Hoang-Long Du , Cuong Ky Nguyen , Bryan H. R. Suryanto , Alexandr N. Simonov , Douglas R. MacFarlane
|
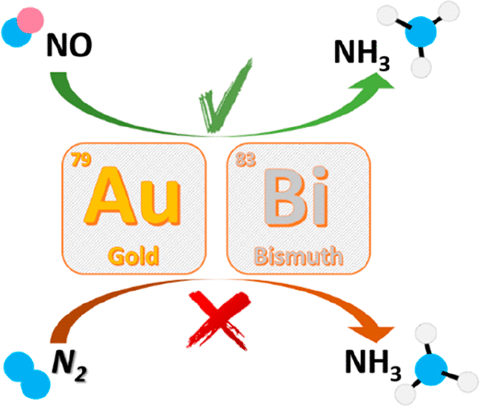
The results presented herein unambiguously demonstrate that neither nanoparticulate carbon-supported gold, nor bismuth powder are active catalysts for the electrocatalytic dinitrogen reduction, but both can efficiently catalyze the electroreduction of NO2–, NO3–, NO, and NO2 to ammonia in an aqueous electrolyte solution. Importantly, reduction of gaseous oxidized forms of nitrogen to NH3 is more facile and occurs at significantly less negative potentials than that of nitrate and nitrite anions.
中文翻译:

硝酸盐,亚硝酸盐和气态氮氧化物的电还原:氮还原研究中氨的潜在来源
结果呈现的这里明确地表明,无论是纳米颗粒的碳载金,也不铋粉末是电催化二氮还原活性的催化剂,但两者都可以有效地催化NO的电还原2 -,NO 3 -,NO,和NO 2,以氨电解质水溶液。重要的是,相比于硝酸根和亚硝酸根阴离子,将气态的氧化形式的氮还原为NH 3更容易并且在负电势下显着减少。
更新日期:2020-05-22
中文翻译:

硝酸盐,亚硝酸盐和气态氮氧化物的电还原:氮还原研究中氨的潜在来源
结果呈现的这里明确地表明,无论是纳米颗粒的碳载金,也不铋粉末是电催化二氮还原活性的催化剂,但两者都可以有效地催化NO的电还原2 -,NO 3 -,NO,和NO 2,以氨电解质水溶液。重要的是,相比于硝酸根和亚硝酸根阴离子,将气态的氧化形式的氮还原为NH 3更容易并且在负电势下显着减少。











































 京公网安备 11010802027423号
京公网安备 11010802027423号