当前位置:
X-MOL 学术
›
Cytom. Part A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Image-Based Cell Profiling Enables Quantitative Tissue Microscopy in Gastroenterology.
Cytometry Part A ( IF 2.5 ) Pub Date : 2020-05-23 , DOI: 10.1002/cyto.a.24042 John W Wills 1 , Jack Robertson 1 , Huw D Summers 2 , Michelle Miniter 1 , Claire Barnes 2 , Rachel E Hewitt 1 , Åsa V Keita 3 , Johan D Söderholm 3 , Paul Rees 2, 4 , Jonathan J Powell 1
Cytometry Part A ( IF 2.5 ) Pub Date : 2020-05-23 , DOI: 10.1002/cyto.a.24042 John W Wills 1 , Jack Robertson 1 , Huw D Summers 2 , Michelle Miniter 1 , Claire Barnes 2 , Rachel E Hewitt 1 , Åsa V Keita 3 , Johan D Söderholm 3 , Paul Rees 2, 4 , Jonathan J Powell 1
Affiliation
|
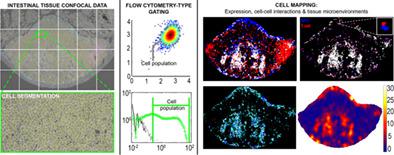
Immunofluorescence microscopy is an essential tool for tissue‐based research, yet data reporting is almost always qualitative. Quantification of images, at the per‐cell level, enables “flow cytometry‐type” analyses with intact locational data but achieving this is complex. Gastrointestinal tissue, for example, is highly diverse: from mixed‐cell epithelial layers through to discrete lymphoid patches. Moreover, different species (e.g., rat, mouse, and humans) and tissue preparations (paraffin/frozen) are all commonly studied. Here, using field‐relevant examples, we develop open, user‐friendly methodology that can encompass these variables to provide quantitative tissue microscopy for the field. Antibody‐independent cell labeling approaches, compatible across preparation types and species, were optimized. Per‐cell data were extracted from routine confocal micrographs, with semantic machine learning employed to tackle densely packed lymphoid tissues. Data analysis was achieved by flow cytometry‐type analyses alongside visualization and statistical definition of cell locations, interactions and established microenvironments. First, quantification of Escherichia coli passage into human small bowel tissue, following Ussing chamber incubations exemplified objective quantification of rare events in the context of lumen‐tissue crosstalk. Second, in rat jejenum, precise histological context revealed distinct populations of intraepithelial lymphocytes between and directly below enterocytes enabling quantification in context of total epithelial cell numbers. Finally, mouse mononuclear phagocyte—T cell interactions, cell expression and significant spatial cell congregations were mapped to shed light on cell–cell communication in lymphoid Peyer's patch. Accessible, quantitative tissue microscopy provides a new window‐of‐insight to diverse questions in gastroenterology. It can also help combat some of the data reproducibility crisis associated with antibody technologies and over‐reliance on qualitative microscopy. © 2020 The Authors. Cytometry Part A published by Wiley Periodicals LLC. on behalf of International Society for Advancement of Cytometry.
中文翻译:

基于图像的细胞分析使胃肠病学中的定量组织显微镜成为可能。
免疫荧光显微镜是基于组织的研究的重要工具,但数据报告几乎总是定性的。在每个细胞级别对图像进行量化,可以使用完整的位置数据进行“流式细胞术类型”分析,但实现这一点很复杂。例如,胃肠组织是高度多样化的:从混合细胞上皮层到离散的淋巴斑块。此外,通常研究不同的物种(例如,大鼠、小鼠和人类)和组织制剂(石蜡/冷冻)。在这里,使用与领域相关的例子,我们开发了开放的、用户友好的方法,可以包含这些变量,为该领域提供定量的组织显微镜。优化了与抗体无关的细胞标记方法,在制备类型和物种之间兼容。每个细胞的数据是从常规共聚焦显微照片中提取的,语义机器学习用于处理密集的淋巴组织。数据分析是通过流式细胞术类型分析以及细胞位置、相互作用和已建立的微环境的可视化和统计定义来实现的。一、量化大肠杆菌进入人类小肠组织,在 Ussing 室孵化之后,举例说明了在管腔 - 组织串扰的背景下对罕见事件的客观量化。其次,在大鼠空肠中,精确的组织学背景揭示了肠细胞之间和直接下方的不同上皮内淋巴细胞群,从而能够在总上皮细胞数量的背景下进行量化。最后,小鼠单核吞噬细胞-T 细胞相互作用、细胞表达和显着的空间细胞聚集被绘制出来,以阐明淋巴淋巴集结中的细胞-细胞通讯。可访问的定量组织显微镜为胃肠病学中的各种问题提供了新的见解。它还可以帮助解决与抗体技术和过度依赖定性显微镜相关的一些数据可重复性危机。由 Wiley Periodicals LLC 出版的Cytometry Part A。代表国际细胞计量学促进会。
更新日期:2020-05-23
中文翻译:

基于图像的细胞分析使胃肠病学中的定量组织显微镜成为可能。
免疫荧光显微镜是基于组织的研究的重要工具,但数据报告几乎总是定性的。在每个细胞级别对图像进行量化,可以使用完整的位置数据进行“流式细胞术类型”分析,但实现这一点很复杂。例如,胃肠组织是高度多样化的:从混合细胞上皮层到离散的淋巴斑块。此外,通常研究不同的物种(例如,大鼠、小鼠和人类)和组织制剂(石蜡/冷冻)。在这里,使用与领域相关的例子,我们开发了开放的、用户友好的方法,可以包含这些变量,为该领域提供定量的组织显微镜。优化了与抗体无关的细胞标记方法,在制备类型和物种之间兼容。每个细胞的数据是从常规共聚焦显微照片中提取的,语义机器学习用于处理密集的淋巴组织。数据分析是通过流式细胞术类型分析以及细胞位置、相互作用和已建立的微环境的可视化和统计定义来实现的。一、量化大肠杆菌进入人类小肠组织,在 Ussing 室孵化之后,举例说明了在管腔 - 组织串扰的背景下对罕见事件的客观量化。其次,在大鼠空肠中,精确的组织学背景揭示了肠细胞之间和直接下方的不同上皮内淋巴细胞群,从而能够在总上皮细胞数量的背景下进行量化。最后,小鼠单核吞噬细胞-T 细胞相互作用、细胞表达和显着的空间细胞聚集被绘制出来,以阐明淋巴淋巴集结中的细胞-细胞通讯。可访问的定量组织显微镜为胃肠病学中的各种问题提供了新的见解。它还可以帮助解决与抗体技术和过度依赖定性显微镜相关的一些数据可重复性危机。由 Wiley Periodicals LLC 出版的Cytometry Part A。代表国际细胞计量学促进会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号