当前位置:
X-MOL 学术
›
Phytochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Alkane-, alkene-, alkyne-γ-lactones and ryanodane diterpenes from aeroponically grown Persea indica roots
Phytochemistry ( IF 3.2 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.phytochem.2020.112398 Braulio M Fraga 1 , Carmen E Díaz 1 , Patricia Bolaños 1 , María Bailén 2 , María Fe Andrés 2 , Azucena González-Coloma 2
Phytochemistry ( IF 3.2 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.phytochem.2020.112398 Braulio M Fraga 1 , Carmen E Díaz 1 , Patricia Bolaños 1 , María Bailén 2 , María Fe Andrés 2 , Azucena González-Coloma 2
Affiliation
|
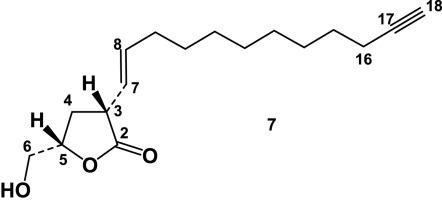
This work presents the study of the roots of the Macaronesian paleoendemism Persea indica (L.) Spreng. The root biomass of this protected tree species has been produced by soil-less aeroponic culture under controlled environment. This system has important advantages over traditional plant production techniques because it provides opportunities to optimize the yield of metabolite production under well-controlled conditions, thereby facilitating commercial-scale production of bioactive compounds. Thus, for the first time a study of this type has permitted the isolation from the roots of seven undescribed dextrorotatory lactones: the alkane-γ-lactones (+)-majoranolide and (+)-dihydromajorenolide, the alkene-γ-lactones (+)-majorenolide and (+)-majorenolide acetate, and the alkyne-γ-lactones, (+)-majorynolide, (+)-majorynolide acetate and (+)-isomajorynolide. In addition, thirteen known compounds were also isolated including two possible avocadofurane precursors, avocadynone acetate and avocadenone acetate, the monoterpene esters cis- and trans-p-coumarate of (-)-borneol, and the ryanoid diterpenes cinnzeylanone, anhidrocinnzeylanine, cinnzeylanine, cinnzeylanol, epiryanodol, perseanol, cinncassiol E, perseaindicol and secoperseanol. The configuration at C-14 de two ryanodane diterpenes has also been revised in this work. Furthermore, (-)-borneol cis-p-coumarate has showed to be insecticidal to S. littoralis and cytotoxic to insect (Sf9) cells, (+)-majorenolide antifeedant to aphids and cytotoxic to Sf9, cinnceylanol antifeedant and insecticidal to S. littoralis, and (+)-majorynolide (2), insecticidal against S. littoralis, cytotoxic to Sf9 and nematicidal, suggesting a defensive role for these compounds.
中文翻译:

来自气培法种植的 Persea indica 根的烷烃-、烯烃-、炔烃-γ-内酯和兰丹二萜
这项工作介绍了对 Macaronesian 古地方性 Persea indica (L.) Spreng 的根源的研究。这种受保护树种的根系生物量是在受控环境下通过无土气培培养产生的。与传统植物生产技术相比,该系统具有重要优势,因为它提供了在良好控制的条件下优化代谢产物产量的机会,从而促进生物活性化合物的商业规模生产。因此,此类研究首次允许从七种未描述的右旋内酯的根中分离:烷烃-γ-内酯 (+)-majorenolide 和 (+)-dihydromajorenolide,烯烃-γ-内酯 (+ )-majorenolide 和 (+)-majorenolide 醋酸盐,以及炔烃-γ-内酯,(+)-majorynolide,(+)-majorynolide 醋酸盐和 (+)-isomajorynolide。此外,还分离了 13 种已知化合物,包括两种可能的鳄梨呋喃前体,醋酸鳄梨酮和醋酸鳄梨酮,(-)-冰片的顺式和反式-对香豆酸酯单萜酯,以及花青素二萜类辛烯酮、去水辛烯胺、辛烯胺、辛烯醇、epryanodol、perseanol、cinncassiol E、perseaindicol 和 secoperseanol。在这项工作中,C-14 de 两个兰丹二萜的配置也进行了修改。此外,(-)-冰片顺-对-香豆酸酯已显示对 S. littoralis 具有杀虫作用,对昆虫 (Sf9) 细胞具有细胞毒性,(+)-majorenolide 对蚜虫具有拒食性,对 Sf9 具有细胞毒性,桂皮醇拒食性对 S. littoralis 具有杀虫作用。 littoralis 和 (+)-majorynolide (2),对 S. littoralis 具有杀虫作用,
更新日期:2020-08-01
中文翻译:

来自气培法种植的 Persea indica 根的烷烃-、烯烃-、炔烃-γ-内酯和兰丹二萜
这项工作介绍了对 Macaronesian 古地方性 Persea indica (L.) Spreng 的根源的研究。这种受保护树种的根系生物量是在受控环境下通过无土气培培养产生的。与传统植物生产技术相比,该系统具有重要优势,因为它提供了在良好控制的条件下优化代谢产物产量的机会,从而促进生物活性化合物的商业规模生产。因此,此类研究首次允许从七种未描述的右旋内酯的根中分离:烷烃-γ-内酯 (+)-majorenolide 和 (+)-dihydromajorenolide,烯烃-γ-内酯 (+ )-majorenolide 和 (+)-majorenolide 醋酸盐,以及炔烃-γ-内酯,(+)-majorynolide,(+)-majorynolide 醋酸盐和 (+)-isomajorynolide。此外,还分离了 13 种已知化合物,包括两种可能的鳄梨呋喃前体,醋酸鳄梨酮和醋酸鳄梨酮,(-)-冰片的顺式和反式-对香豆酸酯单萜酯,以及花青素二萜类辛烯酮、去水辛烯胺、辛烯胺、辛烯醇、epryanodol、perseanol、cinncassiol E、perseaindicol 和 secoperseanol。在这项工作中,C-14 de 两个兰丹二萜的配置也进行了修改。此外,(-)-冰片顺-对-香豆酸酯已显示对 S. littoralis 具有杀虫作用,对昆虫 (Sf9) 细胞具有细胞毒性,(+)-majorenolide 对蚜虫具有拒食性,对 Sf9 具有细胞毒性,桂皮醇拒食性对 S. littoralis 具有杀虫作用。 littoralis 和 (+)-majorynolide (2),对 S. littoralis 具有杀虫作用,











































 京公网安备 11010802027423号
京公网安备 11010802027423号