International Journal of Hydrogen Energy ( IF 7.2 ) Pub Date : 2020-05-23 , DOI: 10.1016/j.ijhydene.2020.04.129 Akari Narayama Sosa , Francisco de Santiago , Álvaro Miranda , Alejandro Trejo , Fernando Salazar , Luis Antonio Pérez , Miguel Cruz-Irisson
|
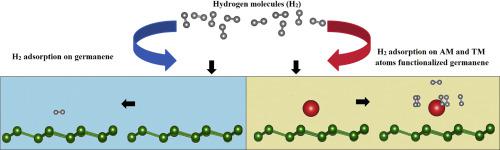
In this work, we have performed density functional theory-based calculations to study the adsorption of H2 molecules on germanene decorated with alkali atoms (AM) and transition metal atoms (TM). The cohesive energy indicates that interaction between AM (TM) atoms and germanene is strong. The values of the adsorption energies of H2 molecules on the AM or TM atoms are in the range physisorption. The K-decorated germanene has the largest storage capacity, being able to bind up to six H2 molecules, whereas the Au and Na atoms adsorbed five and four H2 molecules, respectively. Li and Ag atoms can bind a maximum of three H2 molecules, while Cu-decorated germanene only adsorbed one H2 molecule. Formation energies show that all the studied cases of H2 molecules adsorbed on AM and TM atom-decorated germanene are energetically favorable. These results indicate that decorated germanene can serve as a hydrogen storage system.
中文翻译:

用于储氢的碱金属和过渡金属原子官能化的锗烯:DFT研究
在这项工作中,我们已经进行了基于密度泛函理论的计算,以研究H 2分子在装饰有碱原子(AM)和过渡金属原子(TM)的锗烯上的吸附。内聚能表明AM(TM)原子与锗烯之间的相互作用很强。H 2分子在AM或TM原子上的吸附能的值在物理吸附范围内。K修饰的锗烯具有最大的存储容量,最多可以结合六个H 2分子,而Au和Na原子分别吸附五个和四个H 2分子。Li和Ag原子最多可以结合三个H 2分子,而用Cu装饰的锗烯仅吸附一个H 2分子。形成能表明,所有研究的案例表明,吸附在AM和TM原子修饰的锗烯上的H 2分子在能量上都是有利的。这些结果表明装饰的锗烯可以用作储氢系统。



























 京公网安备 11010802027423号
京公网安备 11010802027423号