Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2020-05-23 , DOI: 10.1016/j.bmc.2020.115560 Jiale Tian 1 , Yingfang He 1 , Winnie Deuther-Conrad 2 , Hualong Fu 1 , Fang Xie 3 , Ying Zhang 1 , Tao Wang 1 , Xiaojun Zhang 4 , Jinming Zhang 4 , Peter Brust 2 , Yiyun Huang 5 , Hongmei Jia 1
|
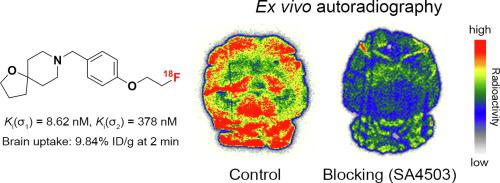
We report the design, synthesis, and evaluation of a series of 1-oxa-8-azaspiro[4.5]decane and 1,5-dioxa-9-azaspiro[5.5]undecane derivatives as selective σ1 receptor ligands. All seven ligands exhibited nanomolar affinity for σ1 receptors (Ki(σ1) = 0.61 – 12.0 nM) and moderate selectivity toward σ2 receptors (Ki(σ2)/ Ki(σ1) = 2 – 44). Compound 8, with the best selectivity among these ligands, was selected for radiolabeling and further evaluation. Radioligand [18F]8 was prepared via nucleophilic 18F-substitution of the corresponding tosylate precursor, with an overall isolated radiochemical yield of 12-35%, a radiochemical purity of >99%, and molar activity of 94 – 121 GBq/μmol. Biodistribution studies of [18F]8 in mice demonstrated high initial brain uptake at 2 min. Pretreatment with SA4503 resulted in significantly reduced brain-to-blood ratio (70% - 75% at 30 min). Ex vivo autoradiography in ICR mice demonstrated high accumulation of the radiotracer in σ1 receptor-rich brain areas. These findings suggest that [18F]8 could be a lead compound for further structural modification to develop potential brain imaging agent for σ1 receptors.
中文翻译:

作为 sigma-1 受体候选放射性配体的新型 1-oxa-8-azaspiro[4.5]decane 衍生物的合成和评估。
我们报告了一系列作为选择性 σ 1受体配体的 1-oxa-8-azaspiro[4.5]decane 和 1,5-dioxa-9-azaspiro[5.5]unedecane 衍生物的设计、合成和评估。所有七个配体均表现出对 σ 1受体的纳摩尔亲和力 ( K i (σ 1 ) = 0.61 – 12.0 nM) 和对 σ 2受体的中等选择性 ( K i (σ 2 )/ K i (σ 1 ) = 2 – 44)。在这些配体中具有最佳选择性的化合物8被选择用于放射性标记和进一步评估。放射性配体[ 18 F] 8通过相应甲苯磺酸盐前体的亲核18 F-取代制备,总分离放射化学收率为 12-35%,放射化学纯度 >99%,摩尔活度为 94 – 121 GBq/μmol 。 [ 18 F] 8在小鼠体内的生物分布研究表明,2 分钟后大脑初始摄取量较高。 SA4503 预处理导致脑血比显着降低(30 分钟时为 70% - 75%)。 ICR 小鼠的离体放射自显影显示放射性示踪剂在富含 σ 1受体的大脑区域中大量积累。这些发现表明,[ 18 F] 8可能是进一步结构修饰的先导化合物,以开发 σ 1受体的潜在脑成像剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号