当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
General Method for Determining Redox Potentials without Electrolyte.
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-05-21 , DOI: 10.1021/acs.jpca.0c02948 Matthew J Bird 1 , Matthew A Pearson 2 , Sadayuki Asaoka 3 , John R Miller 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-05-21 , DOI: 10.1021/acs.jpca.0c02948 Matthew J Bird 1 , Matthew A Pearson 2 , Sadayuki Asaoka 3 , John R Miller 1
Affiliation
|
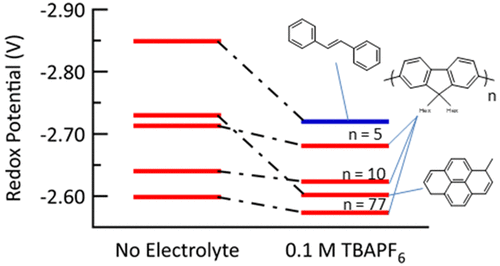
A novel method to determine redox potentials without electrolyte is presented. The method is based on a new ability to determine the dissociation constant, K°d, for ion pairs formed between any radical anion and any inert electrolyte counterion. These dissociation constants can be used to determine relative shifts of redox potential as a function of electrolyte concentration, connecting referenced potentials determined with electrochemistry (with 0.1 M electrolyte) to electrolyte-free values. Pulse radiolysis created radical anions enabling determination of equilibrium constants for electron transfer between anions of donor and acceptor molecules as a function of electrolyte concentration in THF. The measurements determined “composite equilibrium constants”, KeqC, which contain information about the dissociation constant for the electrolyte cations, X+, with the radical anions of both the donor, K°d(D–•,X+) and the acceptor, K°d(A–•,X+). Dissociation constants were obtained for a selection of radical anions with tetrabutylammonium (TBA+). The electrolyte was found to shift the reduction potentials of small molecules 1-methylpyrene and trans-stilbene by close to +130 mV whereas oligo-fluorenes and polyfluorenes experienced shifts of only (+25 ± 6) mV due to charge delocalization weakening the ion pair. These shifts for reduction of aromatic hydrocarbon molecules are smaller than shifts of +232 and +451 mV seen previously for benzophenone radical anion with TBA+ and Na+ respectively where the charge on the radical anion is localized largely on one C═O bond, thus forming a more tightly bound ion pair.
中文翻译:

无电解质测定氧化还原电势的通用方法。
提出了一种无需电解质即可确定氧化还原电势的新方法。该方法基于一种新的确定离解常数K ° d的新能力,该离解常数K ° d为任何自由基阴离子和任何惰性电解质抗衡离子之间形成的离子对。这些解离常数可用于确定氧化还原电势作为电解质浓度的函数的相对位移,并将通过电化学法(使用0.1 M电解质)确定的参考电势与无电解质值联系起来。脉冲辐射分解产生自由基阴离子,从而能够确定供体分子和受体分子之间的阴离子之间电子转移的平衡常数,该常数是THF中电解质浓度的函数。测量确定“复合平衡常数”,KeqC,包含有关电解质阳离子X +与供体K ° d(D –•,X +)和受体K ° d(A –•,X)的自由基阴离子的解离常数的信息+)。获得离解常数,以选择具有四丁基铵(TBA +)的自由基阴离子。发现电解质改变了小分子1-甲基py和反式的还原电位-二苯乙烯的浓度接近+130 mV,而低聚芴和聚芴的迁移仅是(+25±6)mV,这是由于电荷离域作用削弱了离子对。这些还原芳香烃分子的位移小于先前分别用TBA +和Na +产生的二苯甲酮自由基阴离子的+232和+451 mV的位移,其中自由基阴离子上的电荷主要位于一个C═O键上,因此形成更紧密结合的离子对。
更新日期:2020-07-02
中文翻译:

无电解质测定氧化还原电势的通用方法。
提出了一种无需电解质即可确定氧化还原电势的新方法。该方法基于一种新的确定离解常数K ° d的新能力,该离解常数K ° d为任何自由基阴离子和任何惰性电解质抗衡离子之间形成的离子对。这些解离常数可用于确定氧化还原电势作为电解质浓度的函数的相对位移,并将通过电化学法(使用0.1 M电解质)确定的参考电势与无电解质值联系起来。脉冲辐射分解产生自由基阴离子,从而能够确定供体分子和受体分子之间的阴离子之间电子转移的平衡常数,该常数是THF中电解质浓度的函数。测量确定“复合平衡常数”,KeqC,包含有关电解质阳离子X +与供体K ° d(D –•,X +)和受体K ° d(A –•,X)的自由基阴离子的解离常数的信息+)。获得离解常数,以选择具有四丁基铵(TBA +)的自由基阴离子。发现电解质改变了小分子1-甲基py和反式的还原电位-二苯乙烯的浓度接近+130 mV,而低聚芴和聚芴的迁移仅是(+25±6)mV,这是由于电荷离域作用削弱了离子对。这些还原芳香烃分子的位移小于先前分别用TBA +和Na +产生的二苯甲酮自由基阴离子的+232和+451 mV的位移,其中自由基阴离子上的电荷主要位于一个C═O键上,因此形成更紧密结合的离子对。











































 京公网安备 11010802027423号
京公网安备 11010802027423号