当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stabilization of Near-Infrared Fluorescent Proteins by Packaging in Virus-like Particles.
Biomacromolecules ( IF 5.5 ) Pub Date : 2020-05-22 , DOI: 10.1021/acs.biomac.0c00362 Soumen Das 1 , Liangjun Zhao 1 , Stephen N Crooke 1 , Lily Tran 2 , Sonia Bhattacharya 1 , Eric A Gaucher 2 , M G Finn 1, 3
Biomacromolecules ( IF 5.5 ) Pub Date : 2020-05-22 , DOI: 10.1021/acs.biomac.0c00362 Soumen Das 1 , Liangjun Zhao 1 , Stephen N Crooke 1 , Lily Tran 2 , Sonia Bhattacharya 1 , Eric A Gaucher 2 , M G Finn 1, 3
Affiliation
|
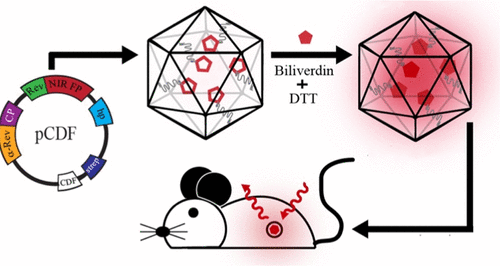
Near-IR fluorescent Qβ virus-like particles (VLPs) were produced in a high yield by packaging highly red-shifted monomeric and dimeric versions of biliverdin-dependent fluorescent proteins within the capsid shell. The simple addition of biliverdin hydrochloride to the medium during or after Escherichia coli protein expression was enough to produce fully matured encapsidated fluorophores. The packaged near-IR proteins exhibited identical photochemical properties to their nonencapsidated analogues but were far more stable toward heat, chaotrope-induced denaturation, and proteolysis. Noninvasive in vivo imaging showed the VLPs to traffic primarily to the liver after systemic injection in mice, revealing that the particles were easily detected by a standard instrument.
中文翻译:

通过包装在病毒样颗粒中来稳定近红外荧光蛋白。
通过在衣壳中包装高胆红移的Biliverdin依赖性荧光蛋白的单体和二聚体形式,可以高产量生产近红外荧光Qβ病毒样颗粒(VLP)。在大肠杆菌蛋白表达期间或之后,将盐酸胆绿素简单地添加到培养基中就足以产生完全成熟的衣壳化的荧光团。包装的近红外蛋白与其非衣壳化的类似物表现出相同的光化学性质,但对热,离液剂诱导的变性和蛋白水解则更加稳定。无创体内成像显示,在小鼠全身注射后,VLP主要转运至肝脏,这表明颗粒可以通过标准仪器轻松检测到。
更新日期:2020-05-22
中文翻译:

通过包装在病毒样颗粒中来稳定近红外荧光蛋白。
通过在衣壳中包装高胆红移的Biliverdin依赖性荧光蛋白的单体和二聚体形式,可以高产量生产近红外荧光Qβ病毒样颗粒(VLP)。在大肠杆菌蛋白表达期间或之后,将盐酸胆绿素简单地添加到培养基中就足以产生完全成熟的衣壳化的荧光团。包装的近红外蛋白与其非衣壳化的类似物表现出相同的光化学性质,但对热,离液剂诱导的变性和蛋白水解则更加稳定。无创体内成像显示,在小鼠全身注射后,VLP主要转运至肝脏,这表明颗粒可以通过标准仪器轻松检测到。











































 京公网安备 11010802027423号
京公网安备 11010802027423号