当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Investigations into the Antibacterial Mechanism of Action of Viridicatumtoxins.
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2020-05-21 , DOI: 10.1021/acsinfecdis.0c00031 Weijia Li 1 , Li Li 2 , Chao Zhang 3 , Yuanheng Cai 4 , Qiyu Gao 1 , Fulin Wang 1 , Yu Cao 1 , Jinzhong Lin 2 , Jie Li 5 , Zhuo Shang 1, 5 , Wei Lin 1, 6
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2020-05-21 , DOI: 10.1021/acsinfecdis.0c00031 Weijia Li 1 , Li Li 2 , Chao Zhang 3 , Yuanheng Cai 4 , Qiyu Gao 1 , Fulin Wang 1 , Yu Cao 1 , Jinzhong Lin 2 , Jie Li 5 , Zhuo Shang 1, 5 , Wei Lin 1, 6
Affiliation
|
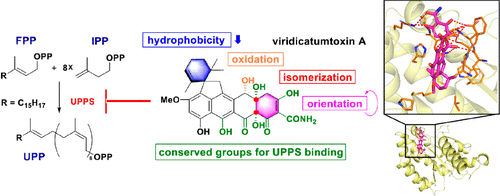
Viridicatumtoxins are a rare class of tetracycline-like antibiotics that strongly inhibit drug-resistant Gram-positive bacteria. Although reported to exhibit in vitro inhibition activity to undecaprenyl pyrophosphate synthase (UPPS), an essential enzyme in bacterial cell wall synthesis, the biological targets and mechanism of action of viridicatumtoxins, especially the drug–target interactions, remain largely unknown. In this study, the structure of Enterococcus faecalis UPPS (EfaUPPS) was first determined, uncovering that EfaUPPS can form not only a typical functional dimer but also an unexpected atypical dimer. We then observed that viridicatumtoxins A (VirA) and B (VirB) are able to bind to UPPSs of E. faecalis, S. aureus, and E. coli in a direct and high-affinity manner as evidenced by in vitro enzyme inhibition assay, surface plasmon resonance (SPR) binding analysis, and in vivo growth inhibition assay, demonstrating that viridicatumtoxins exert antibacterial effects through UPPS binding. The key amino acid residues involved in the interactions with VirA and VirB in EfaUPPS binding pocket were revealed by molecular docking studies, and further validated by site-directed mutagenesis. A single mutation of EfaUPPS at D29A, N31A, and R42A can obviously increase their affinities to VirA, while a single mutation at W228A conferred significant resistance to VirA. Moreover, translation inhibition assay showed that VirA and VirB can weakly inhibit E. coli 70S ribosome. The weak inhibition of ribosome was proposed to be attributed to steric hindrance between viridicatumtoxin ring F and 70S ribosome helix 34 by molecular docking study. Our structural, biochemical, and computational investigations on the interactions of viridicatumtoxins with UPPS and 70S ribosome not only disclosed the potential biological targets of viridicatumtoxins, but also provided a theoretical basis for structural optimization to make new viridicatumtoxin derivatives with improved antimicrobial activities.
中文翻译:

Viridicatumtoxins 的抗菌作用机制的研究。
Viridicatumtoxins 是一种罕见的类四环素类抗生素,可强烈抑制耐药革兰氏阳性菌。尽管据报道对十一烯焦磷酸合酶 (UPPS) 表现出体外抑制活性,UPPS 是细菌细胞壁合成中的一种必需酶,但绿藻毒素的生物学靶标和作用机制,尤其是药物-靶标相互作用,在很大程度上仍是未知的。本研究首次确定了粪肠球菌UPPS(Efa UPPS)的结构,揭示了Efa UPPS不仅可以形成典型的功能性二聚体,还可以形成意想不到的非典型二聚体。然后我们观察到绿色毒素 A (VirA) 和 B (VirB) 能够与粪肠球菌的 UPPS 结合体外酶抑制试验、表面等离子共振 (SPR) 结合分析和体内生长抑制试验证明,绿色葡萄球菌毒素通过UPPS发挥抗菌作用捆绑。通过分子对接研究揭示了与Efa UPPS 结合口袋中的 VirA 和 VirB 相互作用的关键氨基酸残基,并通过定点诱变进一步验证。Efa的单一突变D29A、N31A 和 R42A 的 UPPS 可以明显增加它们对 VirA 的亲和力,而 W228A 的单个突变赋予了对 VirA 的显着抗性。此外,翻译抑制试验表明,VirA和VirB对大肠杆菌70S核糖体有微弱的抑制作用。通过分子对接研究,提出核糖体的弱抑制是由于viridicatumtoxin环F和70S核糖体螺旋34之间的空间位阻。我们对绿藻毒素与 UPPS 和 70S 核糖体相互作用的结构、生化和计算研究不仅揭示了绿藻毒素的潜在生物学靶点,而且为结构优化以制备具有更高抗菌活性的新绿藻毒素衍生物提供了理论基础。
更新日期:2020-07-10
中文翻译:

Viridicatumtoxins 的抗菌作用机制的研究。
Viridicatumtoxins 是一种罕见的类四环素类抗生素,可强烈抑制耐药革兰氏阳性菌。尽管据报道对十一烯焦磷酸合酶 (UPPS) 表现出体外抑制活性,UPPS 是细菌细胞壁合成中的一种必需酶,但绿藻毒素的生物学靶标和作用机制,尤其是药物-靶标相互作用,在很大程度上仍是未知的。本研究首次确定了粪肠球菌UPPS(Efa UPPS)的结构,揭示了Efa UPPS不仅可以形成典型的功能性二聚体,还可以形成意想不到的非典型二聚体。然后我们观察到绿色毒素 A (VirA) 和 B (VirB) 能够与粪肠球菌的 UPPS 结合体外酶抑制试验、表面等离子共振 (SPR) 结合分析和体内生长抑制试验证明,绿色葡萄球菌毒素通过UPPS发挥抗菌作用捆绑。通过分子对接研究揭示了与Efa UPPS 结合口袋中的 VirA 和 VirB 相互作用的关键氨基酸残基,并通过定点诱变进一步验证。Efa的单一突变D29A、N31A 和 R42A 的 UPPS 可以明显增加它们对 VirA 的亲和力,而 W228A 的单个突变赋予了对 VirA 的显着抗性。此外,翻译抑制试验表明,VirA和VirB对大肠杆菌70S核糖体有微弱的抑制作用。通过分子对接研究,提出核糖体的弱抑制是由于viridicatumtoxin环F和70S核糖体螺旋34之间的空间位阻。我们对绿藻毒素与 UPPS 和 70S 核糖体相互作用的结构、生化和计算研究不仅揭示了绿藻毒素的潜在生物学靶点,而且为结构优化以制备具有更高抗菌活性的新绿藻毒素衍生物提供了理论基础。









































 京公网安备 11010802027423号
京公网安备 11010802027423号