The Plant Cell ( IF 10.0 ) Pub Date : 2020-08-01 , DOI: 10.1105/tpc.19.00971 Jinggeng Zhou 1, 2 , Xiaoyang Wang 2 , Yunxia He 1 , Tian Sang 3 , Pengcheng Wang 3 , Shaojun Dai 1 , Shuqun Zhang 4 , Xiangzong Meng 5
|
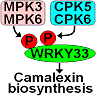
Camalexin is a major phytoalexin that plays a crucial role in disease resistance in Arabidopsis (Arabidopsis thaliana). We previously characterized the regulation of camalexin biosynthesis by the mitogen-activated protein kinases MPK3 and MPK6 and their downstream transcription factor WRKY33. Here, we report that the pathogen-responsive CALCIUM-DEPENDENT PROTEIN KINASE5 (CPK5) and CPK6 also regulate camalexin biosynthesis in Arabidopsis. Chemically induced expression of constitutively active CPK5 or CPK6 variants was sufficient to induce camalexin biosynthesis in transgenic Arabidopsis plants. Consistently, the simultaneous mutation of CPK5 and CPK6 compromised camalexin production in Arabidopsis induced by the fungal pathogen Botrytis cinerea. Moreover, we identified that WRKY33 functions downstream of CPK5/CPK6 to activate camalexin biosynthetic genes, thereby inducing camalexin biosynthesis. CPK5 and CPK6 interact with WRKY33 and phosphorylate its Thr-229 residue, leading to an increase in the DNA binding ability of WRKY33. By contrast, the MPK3/MPK6-mediated phosphorylation of WRKY33 on its N-terminal Ser residues enhances the transactivation activity of WRKY33. Furthermore, both gain- and loss-of-function genetic analyses demonstrated the cooperative regulation of camalexin biosynthesis by CPK5/CPK6 and MPK3/MPK6. Taken together, these findings indicate that WRKY33 functions as a convergent substrate of CPK5/CPK6 and MPK3/MPK6, which cooperatively regulate camalexin biosynthesis via the differential phospho-regulation of WRKY33 activity.
中文翻译:

蛋白激酶 CPK5/CPK6 和 MPK3/MPK6 对转录因子 WRKY33 的差异磷酸化协同调节拟南芥中的 Camalexin 生物合成。
Camalexin 是一种主要的植物抗毒素,在拟南芥 ( Arabidopsis thaliana ) 的抗病性中发挥着至关重要的作用。我们之前描述了丝裂原激活蛋白激酶 MPK3 和 MPK6 及其下游转录因子 WRKY33 对camalexin生物合成的调节。在这里,我们报道了病原体响应性钙依赖性蛋白激酶5 (CPK5) 和CPK6 也调节拟南芥中camalexin 的生物合成。化学诱导的组成型活性 CPK5 或 CPK6 变体的表达足以诱导转基因拟南芥植物中的camalexin生物合成。一致地, CPK5和CPK6的同时突变损害了由真菌病原体灰葡萄孢诱导的拟南芥中camalexin的产生。此外,我们还发现WRKY33在CPK5/CPK6下游发挥作用,激活camalexin生物合成基因,从而诱导camalexin生物合成。 CPK5 和 CPK6 与 WRKY33 相互作用并磷酸化其 Thr-229 残基,导致 WRKY33 的 DNA 结合能力增强。相比之下,MPK3/MPK6 介导的 WRKY33 N 端 Ser 残基磷酸化增强了 WRKY33 的反式激活活性。此外,功能获得和丧失的遗传分析都证明了 CPK5/CPK6 和 MPK3/MPK6 对camalexin生物合成的协同调节。总而言之,这些发现表明 WRKY33 作为 CPK5/CPK6 和 MPK3/MPK6 的聚合底物发挥作用,它们通过 WRKY33 活性的差异磷酸化调节协同调节camalexin 生物合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号