当前位置:
X-MOL 学术
›
Eur. J. Immunol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Relationship between T cell receptor clonotype and PD-1 expression of tumor-infiltrating lymphocytes in colorectal cancer.
European Journal of Immunology ( IF 4.5 ) Pub Date : 2020-05-22 , DOI: 10.1002/eji.201948399 Kenta Sukegawa 1, 2 , Kiyomi Shitaoka 1 , Hiroshi Hamana 1 , Eiji Kobayashi 1 , Yoshihiro Miyahara 3 , Keisuke Fujii 4 , Kei Tsuda 1, 5 , Shiori Saeki 1, 2 , Takuya Nagata 2 , Tatsuhiko Ozawa 1 , Shigeru Saito 5 , Tsutomu Fujii 2 , Atsushi Muraguchi 1 , Hiroshi Shiku 3 , Hiroyuki Kishi 1
European Journal of Immunology ( IF 4.5 ) Pub Date : 2020-05-22 , DOI: 10.1002/eji.201948399 Kenta Sukegawa 1, 2 , Kiyomi Shitaoka 1 , Hiroshi Hamana 1 , Eiji Kobayashi 1 , Yoshihiro Miyahara 3 , Keisuke Fujii 4 , Kei Tsuda 1, 5 , Shiori Saeki 1, 2 , Takuya Nagata 2 , Tatsuhiko Ozawa 1 , Shigeru Saito 5 , Tsutomu Fujii 2 , Atsushi Muraguchi 1 , Hiroshi Shiku 3 , Hiroyuki Kishi 1
Affiliation
|
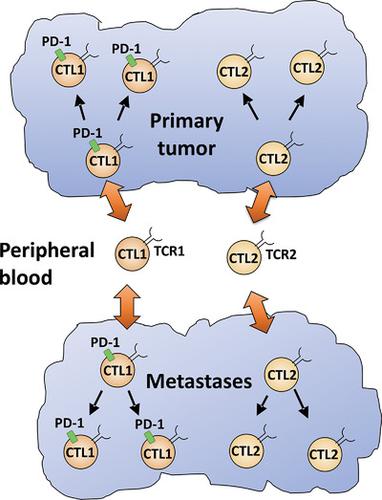
Adoptive T cell therapy using tumor‐specific T cells or TCR‐modified T cells is a promising next‐generation immunotherapy. The major source of tumor‐reactive T cells is PD‐1+ tumor‐infiltrating lymphocytes (TILs). In contrast, PD‐1− TILs have received little attention. Here, we analyzed the TCR‐β repertoires of PD‐1− and PD‐1+ CD8+ TILs derived from colorectal cancer and breast cancer. Approximately 40–60% of the PD‐1+ population consisted of oligoclonal populations in both colorectal cancer and breast cancer. In contrast, approximately 37% of the PD‐1− population consisted of an oligoclonal population in colorectal cancer, whereas 14% of them were oligoclonal in breast cancer. In colorectal cancer, the TCR repertoires of PD‐1−CD8+ TILs and PD‐1+CD8+ TILs hardly overlapped. Interestingly, clonally expanded CD8+ TILs in primary tumors and the metastases expressing the same clonotypic TCR showed the same phenotype regarding the PD‐1‐expression. These results suggest that the intrinsic properties of TCRs determine the fate of TILs in terms of whether they become PD‐1+ or PD‐1− in the tumor microenvironment. Further functional analysis of TCRs in TILs will allow us to better understand the regulatory mechanisms for PD‐1 expression on TILs and may contribute to tumor immunotherapy.
中文翻译:

大肠癌T细胞受体克隆型与肿瘤浸润淋巴细胞PD-1表达的关系。
使用肿瘤特异性T细胞或TCR修饰的T细胞的过继T细胞疗法是一种有前途的下一代免疫疗法。肿瘤反应性T细胞的主要来源是PD-1 +肿瘤浸润淋巴细胞(TIL)。相反,PD-1 − TIL很少受到关注。在这里,我们分析了PD-1的TCR-β剧目-和PD-1 + CD8 + TIL的大肠癌和乳腺癌的。在PD-1 +人群中,约有40-60%的人是结直肠癌和乳腺癌中的寡克隆人群。相反,PD-1的约37%-人群中,大肠癌为寡克隆人群,而乳腺癌为14%。在大肠癌中,PD-1 − CD8 + TIL和PD-1 + CD8 + TIL的TCR谱几乎不重叠。有趣的是,在原发性肿瘤中克隆扩增的CD8 + TIL和表达相同克隆型TCR的转移表现出与PD-1表达相同的表型。这些结果表明,TCR的内在特性决定其是否成为PD-1项肿瘤浸润淋巴细胞的命运+或PD-1 -在肿瘤微环境中。对TIL中TCR的进一步功能分析将使我们能够更好地了解TIL上PD-1表达的调节机制,并可能有助于肿瘤免疫治疗。
更新日期:2020-05-22
中文翻译:

大肠癌T细胞受体克隆型与肿瘤浸润淋巴细胞PD-1表达的关系。
使用肿瘤特异性T细胞或TCR修饰的T细胞的过继T细胞疗法是一种有前途的下一代免疫疗法。肿瘤反应性T细胞的主要来源是PD-1 +肿瘤浸润淋巴细胞(TIL)。相反,PD-1 − TIL很少受到关注。在这里,我们分析了PD-1的TCR-β剧目-和PD-1 + CD8 + TIL的大肠癌和乳腺癌的。在PD-1 +人群中,约有40-60%的人是结直肠癌和乳腺癌中的寡克隆人群。相反,PD-1的约37%-人群中,大肠癌为寡克隆人群,而乳腺癌为14%。在大肠癌中,PD-1 − CD8 + TIL和PD-1 + CD8 + TIL的TCR谱几乎不重叠。有趣的是,在原发性肿瘤中克隆扩增的CD8 + TIL和表达相同克隆型TCR的转移表现出与PD-1表达相同的表型。这些结果表明,TCR的内在特性决定其是否成为PD-1项肿瘤浸润淋巴细胞的命运+或PD-1 -在肿瘤微环境中。对TIL中TCR的进一步功能分析将使我们能够更好地了解TIL上PD-1表达的调节机制,并可能有助于肿瘤免疫治疗。











































 京公网安备 11010802027423号
京公网安备 11010802027423号