当前位置:
X-MOL 学术
›
Biotechnol. Bioeng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Scalable, two-stage, autoinduction of recombinant protein expression in E. coli utilizing phosphate depletion.
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2020-05-22 , DOI: 10.1002/bit.27440 Romel Menacho-Melgar 1 , Zhixia Ye 1 , Eirik A Moreb 1 , Tian Yang 1 , John P Efromson 1 , John S Decker 1 , Ruixin Wang 1 , Michael D Lynch 1
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2020-05-22 , DOI: 10.1002/bit.27440 Romel Menacho-Melgar 1 , Zhixia Ye 1 , Eirik A Moreb 1 , Tian Yang 1 , John P Efromson 1 , John S Decker 1 , Ruixin Wang 1 , Michael D Lynch 1
Affiliation
|
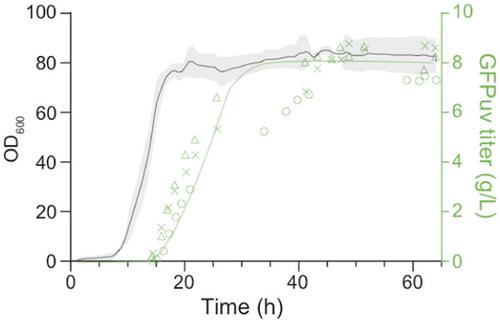
We report the scalable production of recombinant proteins in Escherichia coli, reliant on tightly controlled autoinduction, triggered by phosphate depletion in the stationary phase. The method, reliant on engineered strains and plasmids, enables improved protein expression across scales. Expression levels using this approach have reached as high as 55% of the total cellular protein. The initial use of the method in instrumented fed‐batch fermentations enables cell densities of ∼30 gCDW/L and protein titers up to 8.1 ± 0.7 g/L (∼270 mg/gCDW). The process has also been adapted to an optimized autoinduction media, enabling routine batch production at culture volumes of 20 μl (384‐well plates), 100 μl (96‐well plates), 20 ml, and 100 ml. In batch cultures, cell densities routinely reach ∼5–7 gCDW/L, offering protein titers above 2 g/L. The methodology has been validated with a set of diverse heterologous proteins and is of general use for the facile optimization of routine protein expression from high throughput screens to fed‐batch fermentation.
中文翻译:

利用磷酸盐消耗在大肠杆菌中进行可扩展的两阶段自诱导重组蛋白表达。
我们报告了大肠杆菌中重组蛋白的可扩展生产,依赖于严格控制的自诱导,由固定相中的磷酸盐耗尽触发。该方法依赖于工程菌株和质粒,可以改善跨尺度的蛋白质表达。使用这种方法的表达水平已高达细胞总蛋白的 55%。该方法最初在仪器化补料分批发酵中的应用使细胞密度约为 30 gCDW/L,蛋白质滴度高达 8.1 ± 0.7 g/L(~270 mg/gCDW)。该工艺还适用于优化的自诱导培养基,能够以 20 μl(384 孔板)、100 μl(96 孔板)、20 ml 和 100 ml 的培养体积进行常规批量生产。在分批培养中,细胞密度通常达到 ∼5-7 gCDW/L,提供高于 2 g/L 的蛋白质滴度。
更新日期:2020-05-22
中文翻译:

利用磷酸盐消耗在大肠杆菌中进行可扩展的两阶段自诱导重组蛋白表达。
我们报告了大肠杆菌中重组蛋白的可扩展生产,依赖于严格控制的自诱导,由固定相中的磷酸盐耗尽触发。该方法依赖于工程菌株和质粒,可以改善跨尺度的蛋白质表达。使用这种方法的表达水平已高达细胞总蛋白的 55%。该方法最初在仪器化补料分批发酵中的应用使细胞密度约为 30 gCDW/L,蛋白质滴度高达 8.1 ± 0.7 g/L(~270 mg/gCDW)。该工艺还适用于优化的自诱导培养基,能够以 20 μl(384 孔板)、100 μl(96 孔板)、20 ml 和 100 ml 的培养体积进行常规批量生产。在分批培养中,细胞密度通常达到 ∼5-7 gCDW/L,提供高于 2 g/L 的蛋白质滴度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号