Molecular Therapy - Methods & Clinical Development ( IF 4.6 ) Pub Date : 2020-05-22 , DOI: 10.1016/j.omtm.2020.05.014 Liujiang Song 1, 2, 3 , Jacquelyn J Bower 1, 4 , Telmo Llanga 1 , Jacklyn H Salmon 5 , Matthew L Hirsch 1, 3 , Brian C Gilger 1, 5
|
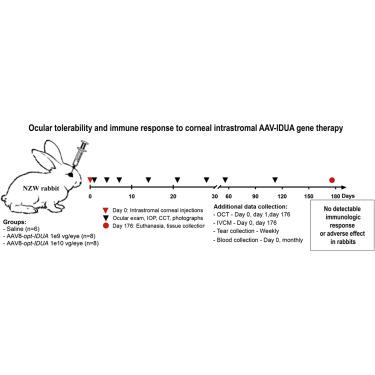
The chronic ocular toxicity, tolerability, and inflammation following corneal intrastromal injection of saline or escalating doses of an adeno-associated virus (AAV) containing a codon-optimized α-l-iduronidase (AAV-opt-IDUA) expression cassette were evaluated in New Zealand White rabbits. Corneal opacity following corneal intrastromal injection resolved by 24 h. Mild elevation of clinical ocular inflammation was observed 24 h after injection, but it returned to baseline by day 7 and no abnormalities were noted through 6 months of observation after injection. Vector genomes and IDUA cDNA were detected in the injected corneas in a dose-dependent manner. Both the lowest administered AAV-opt-IDUA dose, shown to be effective in mucopolysaccharidosis type I (MPS I) dogs, and a 10-fold higher dose of AAV-opt-IDUA resulted in no detectable immunologic response or adverse effect in rabbits. Vector genomes outside of the eye were rarely detected following corneal intrastromal injection of AAV-opt-IDUA, and neutralizing antibodies to the AAV capsid were not present at the experimental conclusion. This study, combined with our previous studies in MPS I dogs, suggests that AAV-opt-IDUA corneal gene therapy following corneal intrastromal injection of AAV-opt-IDUA has the potential to prevent and reverse blindness in MPS I patients in a safe and effective manner.
中文翻译:

新西兰白兔角膜基质内 AAV-IDUA 基因治疗的眼部耐受性和免疫反应。
New 中评估了角膜基质内注射盐水或递增剂量的含有密码子优化的 α- l-艾杜糖醛酸酶 (AAV-opt- IDUA ) 表达盒的腺相关病毒 (AAV) 后的慢性眼毒性、耐受性和炎症。新西兰白兔。角膜基质注射后角膜混浊在 24 小时内消失。注射后24小时观察到临床眼部炎症轻度升高,但到第7天恢复到基线,并且注射后6个月的观察没有发现异常。在注射的角膜中以剂量依赖性方式检测到载体基因组和IDUA cDNA。最低的 AAV-opt- IDUA剂量(显示对 I 型粘多糖贮积症(MPS I)狗有效)和 10 倍高剂量的 AAV-opt- IDUA均未在兔子中导致可检测到的免疫反应或不良反应。角膜基质内注射 AAV-opt- IDUA后,很少检测到眼外的载体基因组,并且实验结论中不存在针对 AAV 衣壳的中和抗体。这项研究与我们之前对 MPS I 犬的研究相结合,表明角膜基质内注射 AAV-opt- IDUA后进行 AAV-opt -IDUA角膜基因治疗有可能安全有效地预防和逆转 MPS I 患者的失明。方式。











































 京公网安备 11010802027423号
京公网安备 11010802027423号