Journal of Photochemistry and Photobiology B: Biology ( IF 3.9 ) Pub Date : 2020-05-22 , DOI: 10.1016/j.jphotobiol.2020.111903 Lucas Henrique de Paula Zago 1 , Sarah Raquel de Annunzio 1 , Kleber Thiago de Oliveira 2 , Paula Aboud Barbugli 3 , Belen Retamal Valdes 4 , Magda Feres 4 , Carla Raquel Fontana 1
|
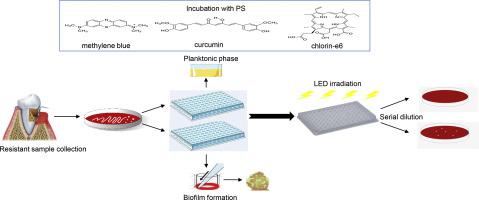
The antimicrobial photodynamic therapy (aPDT) has stood out as an alternative and promising method of disinfection and has been exploited for the treatment of oral bacteria. In this study, we evaluate in vitro the action of aPDT, mediated by methylene blue, chlorin-e6, and curcumin against clinical subgingival plaques that were resistant to metronidazole. The sensitivity profile of the samples to metronidazole was analyzed by the agar dilution method. Cell viability in the planktonic and biofilm phase was assessed by CFU / mL. The composition of the biofilm was evaluated by the checkboard DNA-DNA Hibrydization technique. Photosensitizers internalization was qualitatively assessed by confocal fluorescence microscopy (CLSM). The aPDT mediated by the three photosensitizers tested was able to reduce the totality of the planktonic microbial load and partially reduce the biofilm samples. The analysis performed by CLSM showed that the photosensitizers used in the application of aPDT were able to permeate the interior of the biofilm. The aPDT has been shown to be useful in a supportive and effective approach to the treatment of periodontal disease.
中文翻译:

抗甲硝唑的牙菌斑细菌的抗菌光动力疗法。
抗菌光动力疗法(aPDT)作为一种替代的有前途的消毒方法已脱颖而出,并已被用于口腔细菌的治疗。在这项研究中,我们评估体外飞机乘客离境税的作用下,通过亚甲基蓝,二氢-E6和姜黄素对手是抗临床甲硝唑龈下菌斑介导的。用琼脂稀释法分析样品对甲硝唑的敏感性。通过CFU / mL评估浮游和生物膜相中的细胞活力。通过棋盘DNA-DNA评估生物膜的组成杂化技术。通过共聚焦荧光显微镜(CLSM)定性评估了光敏剂的内在化。由测试的三种光敏剂介导的aPDT能够减少浮游微生物负荷的总量并部分减少生物膜样品。CLSM进行的分析表明,用于aPDT的光敏剂能够渗透到生物膜的内部。已经证明了aPDT在牙周疾病的支持性和有效方法中是有用的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号