Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-05-22 , DOI: 10.1016/j.jmb.2020.05.012 Komal Soni 1 , Santiago Martínez-Lumbreras 1 , Michael Sattler 1
|
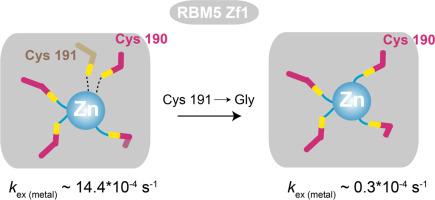
The multi-domain RNA binding protein RBM5 is a molecular signature of metastasis. RBM5 regulates alternative splicing of apoptotic genes including the cell death receptor Fas and the initiator Caspase-2. The RBM5 RanBP2-type zinc finger (Zf1) is known to specifically recognize single-stranded RNAs with high affinity. Here, we study the structure and conformational dynamics of the Zf1 zinc finger of human RBM5 using NMR. We show that the presence of a non-canonical cysteine in Zf1 kinetically destabilizes the protein. Metal-exchange kinetics show that mutation of the cysteine establishes high-affinity coordination of the zinc. Our data indicate that selection of such a structurally destabilizing mutation during the course of evolution could present an opportunity for functional adaptation of the protein.
中文翻译:

RBM5 RanBP2型锌指中锌配位不一致的构象动力学。
多域RNA结合蛋白RBM5是转移的分子标志。RBM5调节凋亡基因的选择性剪接,包括细胞死亡受体Fas和启动子Caspase-2。众所周知,RBM5 RanBP2型锌指(Zf1)能以高亲和力特异性识别单链RNA。在这里,我们使用NMR研究人RBM5的Zf1锌指的结构和构象动力学。我们显示Zf1中的非规范性半胱氨酸的存在会动摇蛋白质的稳定性。金属交换动力学表明,半胱氨酸的突变建立了锌的高亲和力配位。我们的数据表明,在进化过程中选择这种结构不稳定的突变可能为蛋白质的功能适应提供了机会。









































 京公网安备 11010802027423号
京公网安备 11010802027423号