Journal of Advanced Research ( IF 11.4 ) Pub Date : 2020-05-22 , DOI: 10.1016/j.jare.2020.05.012 Anandalakshmi Venkatraman 1 , Minh-Dao Duong-Thi 2 , Konstantin Pervushin 2 , Sten Ohlson 2 , Jodhbir Singh Mehta 3, 4
|
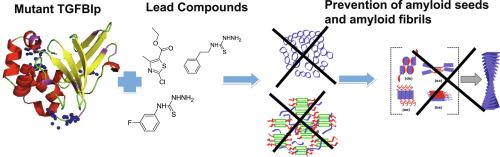
Corneal dystrophies are a group of genetically inherited disorders with mutations in the TGFBI gene affecting the Bowman’s membrane and the corneal stroma. The mutant TGFBIp is highly aggregation-prone and is deposited in the cornea. Depending on the type of mutation the protein deposits may vary (amyloid, amorphous powdery aggregate or a mixed form of both), making the cornea opaque and thereby decreases visual acuity. The aggregation of the mutant protein is found to be specific with a unique aggregation mechanism distinct to the cornea. The proteolytic processing of the mutant protein is reported to be different compared to the WT protein. The proteolytic processing of mutant protein gives rise to highly amyloidogenic peptide fragments. The current treatment option, available for patients, is tissue replacement surgery that is associated with high recurrence rates. The clinical need for a simple treatment option for corneal dystrophy patients has become highly essential either to prevent the protein aggregation or to dissolve the preformed aggregates. Here, we report the screening of 2500 compounds from the Maybridge RO3 fragment library using weak affinity chromatography (WAC). The primary hits from WAC were validated by 15N-HSQC NMR assays and specific regions of binding were identified. The recombinant mutant proteins (4th FAS-1 domain of R555W and H572R) were subjected to limited proteolysis by trypsin together with the lead compounds identified by NMR assays. The lead compounds (MO07617, RJF00203 and, BTB05094) were effective to delay/prevent the generation of amyloidogenic peptides in the R555W mutant and compounds (RJF00203 and BTB05094) were effective to delay/prevent the generation of amyloidogenic peptides in the H572R mutant. Thus the lead compounds reported here upon further validation and/or modification might be proposed as a potential treatment option to prevent/delay aggregation by inhibiting the formation of amyloidogenic peptides in TGFBI-corneal dystrophy.
中文翻译:

转化生长因子β诱导蛋白(TGFBIp)蛋白水解谱的药物调节为TGFBI-角膜营养不良的治疗提供了新途径。
角膜营养不良是TGFBI突变的一组遗传性疾病基因影响鲍曼氏膜和角膜基质。突变体TGFBIp高度易聚集,并沉积在角膜中。取决于突变的类型,蛋白质沉积物可能会变化(淀粉样蛋白,无定形粉末状聚集体或两者的混合形式),使角膜变得不透明,从而降低视力。发现突变蛋白的聚集是特异性的,其独特的聚集机制不同于角膜。据报道,与野生型蛋白质相比,突变型蛋白质的蛋白水解过程不同。突变蛋白的蛋白水解过程产生了高度淀粉样肽片段。对于患者可用的当前治疗选择是与高复发率相关的组织置换手术。对于角膜营养不良患者而言,对简单治疗选择的临床需求对于防止蛋白质聚集或溶解预先形成的聚集体已经变得至关重要。在这里,我们报告使用弱亲和色谱法(WAC)从Maybridge RO3片段库中筛选2500种化合物。WAC的主要命中数据已通过验证鉴定了15种N-HSQC NMR测定法和结合的特定区域。重组突变蛋白(R555W和H572R的第4个FAS-1域)通过胰蛋白酶和通过NMR分析鉴定的先导化合物进行了有限的蛋白水解。前导化合物(MO07617,RJF00203和BTB05094)可有效延迟/预防R555W突变体中淀粉样肽的生成,化合物(RJF00203和BTB05094)可有效延迟/预防H572R突变体中淀粉样肽的生成。因此,在进一步验证和/或修饰后,此处报道的先导化合物可能被提议为通过抑制TGFBI-角膜营养不良症中淀粉样蛋白生成肽的形成来预防/延迟聚集的潜在治疗选择。











































 京公网安备 11010802027423号
京公网安备 11010802027423号